The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics
- PMID: 39091205
- PMCID: PMC11294818
- DOI: 10.1158/2159-8290.CD-24-0002
The Interplay between Extracellular Matrix Remodeling and Cancer Therapeutics
Abstract
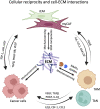
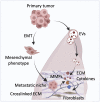
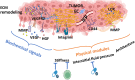
The extracellular matrix (ECM) is an abundant noncellular component of most solid tumors known to support tumor progression and metastasis. The interplay between the ECM and cancer therapeutics opens up new avenues in understanding cancer biology. While the ECM is known to protect the tumor from anticancer agents by serving as a biomechanical barrier, emerging studies show that various cancer therapies induce ECM remodeling, resulting in therapy resistance and tumor progression. This review discusses critical issues in this field including how the ECM influences treatment outcome, how cancer therapies affect ECM remodeling, and the challenges associated with targeting the ECM. Significance: The intricate relationship between the extracellular matrix (ECM) and cancer therapeutics reveals novel insights into tumor biology and its effective treatment. While the ECM may protect tumors from anti-cancer agents, recent research highlights the paradoxical role of therapy-induced ECM remodeling in promoting treatment resistance and tumor progression. This review explores the key aspects of the interplay between ECM and cancer therapeutics.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J. Prakash reports grants from Dutch Cancer Foundation during the conduct of the study; non-financial support from ScarTec Therapeutics outside the submitted work; in addition, J. Prakash has a patent for WO2017069627A1 issued and licensed to ScarTec Therapeutics; and ERC Advanced Grant has been awarded to J. Prakash in 2024 which forms the basis for some perspectives discussed in this review. Y. Shaked reports I founded a company which deals with fibrotic diseases.
Figures


References
-
- Cox TR. The matrix in cancer. Nat Rev Cancer 2021;21:217–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

