Isoflavone Derivatives as Potential Anticancer Agents: Synthesis and Bioactivity Studies
- PMID: 39091268
- PMCID: PMC11617652
- DOI: 10.1002/cmdc.202400420
Isoflavone Derivatives as Potential Anticancer Agents: Synthesis and Bioactivity Studies
Abstract
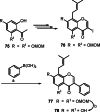
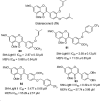
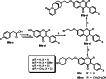
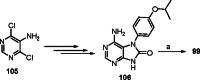
Isoflavones are phenolic natural compounds with a C6C3C6 framework. They possess a plethora of biological activities that are associated with putative benefits to human health. In particular, the cancer chemopreventive and chemotherapeutic potential of isoflavones has attracted the interest of researchers. Several isoflavone derivatives have been synthesised and probed for their anticancer activities. The isoflavone analogues are mainly synthesised by molecular hybridisation and other strategies that enable diversification through early or late-stage functionalisation of A-, B- and C-rings of the isoflavones. This has resulted in the discovery of isoflavone analogues with improved antiproliferative activities against several cancer cells and different mechanisms of action. In this review, the synthesis of isoflavone derivatives and their anticancer activity studies are discussed.
Keywords: Anticancer; Derivatives; Isoflavones; Natural compounds.
© 2024 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The author declares no conflicts of interest.
Figures





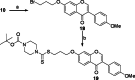

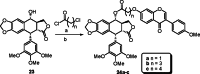






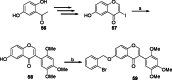


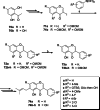


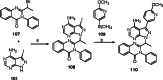
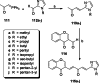

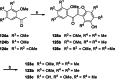

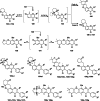

References
-
- Veitch N. C., Nat. Prod. Rep. 2013, 30, 988–1027. - PubMed
-
- Al-Maharik N., Nat. Prod. Rep. 2019, 36, 1156–1195. - PubMed
-
- Tu Y., Wang K., Jia X., Tan L., Han B., Zhang Q., Li Y., He C., J. Agric. Food Chem. 2020, 68, 10664–10677. - PubMed
-
- Çiçek S. S., Galarza Pérez M., Wenzel-Storjohann A., Bezerra R. M., Segovia J. F. O., Girreser U., Kanzaki I., Tasdemir D., J. Nat. Prod. 2022, 85, 927–935. - PubMed
-
- Drewes S. E., Horn M. M., Munro O. Q., Dhlamini J. T. B., Meyer J. J. M., Rakuambo N. C., Phytochemistry 2002, 59, 739–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

