Bioenzymatic detoxification of mycotoxins
- PMID: 39091297
- PMCID: PMC11291262
- DOI: 10.3389/fmicb.2024.1434987
Bioenzymatic detoxification of mycotoxins
Abstract
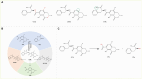
Mycotoxins are secondary metabolites produced during the growth, storage, and transportation of crops contaminated by fungi and are physiologically toxic to humans and animals. Aflatoxin, zearalenone, deoxynivalenol, ochratoxin, patulin, and fumonisin are the most common mycotoxins and can cause liver and nervous system damage, immune system suppression, and produce carcinogenic effects in humans and animals that have consumed contaminated food. Physical, chemical, and biological methods are generally used to detoxify mycotoxins. Although physical methods, such as heat treatment, irradiation, and adsorption, are fast and simple, they have associated problems including incomplete detoxification, limited applicability, and cause changes in food characteristics (e.g., nutritive value, organoleptic properties, and palatability). Chemical detoxification methods, such as ammonification, ozonation, and peroxidation, pollute the environment and produce food safety risks. In contrast, bioenzymatic methods are advantageous as they achieve selective detoxification and are environmentally friendly and reusable; thus, these methods are the most promising options for the detoxification of mycotoxins. This paper reviews recent research progress on common mycotoxins and the enzymatic principles and mechanisms for their detoxification, analyzes the toxicity of the degradation products and describes the challenges faced by researchers in carrying out enzymatic detoxification. In addition, the application of enzymatic detoxification in food and feed is discussed and future directions for the development of enzymatic detoxification methods are proposed for future in-depth study of enzymatic detoxification methods.
Keywords: degradation; detoxification; enzymes; fungi; mechanism; mycotoxins.
Copyright © 2024 Liu, Zhang, Luan, Zhang, Xu, Feng and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

