Gamma-aminobutyric acid treatment promotes resistance against Sogatella furcifera in rice
- PMID: 39091314
- PMCID: PMC11291254
- DOI: 10.3389/fpls.2024.1419999
Gamma-aminobutyric acid treatment promotes resistance against Sogatella furcifera in rice
Abstract
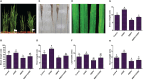
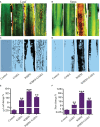
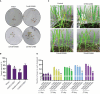
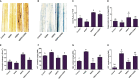
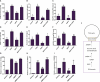
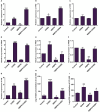
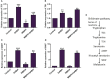
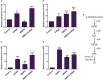
The Sogatella furcifera (Horváth) (Homoptera: Delphacidae) is a white-backed planthopper (WBPH) that causes "hopper burn" in rice, resulting in severe yield loss. Gamma-aminobutyric acid (GABA) is a well-known neurotransmitter that inhibits neurotransmission in insects by binding to specific receptors. In this study, we investigated the potential role of GABA in modulating rice resistance to WBPH and evaluated possible defense mechanisms. The experiment was conducted in green house in pots consist of four groups: control, GABA-treated, WBPH-infested, and WBPH-infested treated with GABA. Among the various tested concentration of GABA, 15 mM GABA was applied as a single treatment in water. The treatment was administered one week before WBPH infestation. The results revealed that 15 mM GABA treatment strongly increased WBPH resistance. A plate-based assay indicated that direct application of 15 mM GABA increased the mortality rate of WBPH and increased the damage recovery rate in rice plants. We found that GABA treatment increased the activation of antioxidant enzymes and reduced the reactive oxygen species content and malondialdehyde contents, and reduced the damage rate caused by WBPH. Interestingly, GABA-supplemented plants infested with WBPH exhibited increased phenylalanine ammonia-lyase and pathogenesis-related (PR) genes expression levels. GABA induced the accumulation of abscisic acid (ABA) and salicylic acid (SA) and enhanced the stomata closure and reduced leaf vessels to reduce water conductance during WBPH stress. Furthermore, we found that GABA application to the plant induced the expression of Jasmonic acid (JA) biosynthesis genes (LOX, AOS, AOC, and OPR) and melatonin biosynthesis-related genes (TDC, T5H, ASMT, and SNAT). Our study suggested that GABA increases resistance against WBPH infestation by regulating antioxidant defense system, TCA cycle regulation, phytohormonal signaling, and PR gene regulation.
Keywords: Sogatella furcifera; antioxidant; gamma-aminobutyric acid; melatonin; phytohormone; tricarboxylic acid cycle.
Copyright © 2024 Jan, Asif, Asaf, Lubna, Khan, Khan and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abd Elbar O. H., Elkelish A., Niedbała G., Farag R., Wojciechowski T., Mukherjee S., et al. (2021). Protective effect of γ-aminobutyric acid against chilling stress during reproductive stage in tomato plants through modulation of sugar metabolism, chloroplast integrity, and antioxidative defense systems. Front. Plant Sci. 12, 663750. doi: 10.3389/fpls.2021.663750 - DOI - PMC - PubMed
-
- Adhikari B., Dhungana S. K., Ali M. W., Adhikari A., Kim I.-D., Shin D.-H. (2019). Antioxidant activities, polyphenol, flavonoid, and amino acid contents in peanut shell. J. Saudi Soc. Agric. Sci. 18, 437–442. doi: 10.1016/j.jssas.2018.02.004 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

