Antibiotic-induced severe cutaneous adverse reactions: a single-center retrospective study over ten years
- PMID: 39091503
- PMCID: PMC11291224
- DOI: 10.3389/fimmu.2024.1415830
Antibiotic-induced severe cutaneous adverse reactions: a single-center retrospective study over ten years
Abstract
Objective: Severe cutaneous adverse reactions (SCARs) are rare but life-threatening, with antibiotics being the main cause. This retrospective study from a single center was designed to analyze the culprit drugs, clinical features and treatment outcomes of antibiotic-induced SCARs.
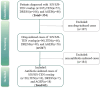
Methods: We analyzed cases of antibiotic-induced SCARs in a tertiary hospital in China between January 2013 and January 2024, including Steven-Johnson syndrome (SJS) or Stevens-Johnson syndrome-toxic epidermal necrolysis (SJS-TEN) overlap, toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) and acute generalized exanthematous pustulosis (AGEP). Descriptive analysis of the demographic characteristics, clinical manifestations, treatment and prognosis were carried out.
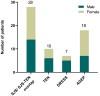
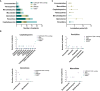
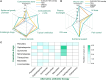
Results: Among 354 cases of SCARs, 63 validated antibiotic-related cases were included. Cephalosporins (31.7%), penicillins (25.4%), and quinolones (19.0%) were the most common triggers for SCARs. Overall, liver (50.8%), lungs (31.7%), and kidneys (23.8%) were the most frequently affected organ in SCARs cases. Eight patients (28.6%) in the SJS/SJS-TEN overlap group and 8 patients (80.0%) in the TEN group received combination therapy of corticosteroids and IVIG. Patients with SCARs caused by penicillins or cephalosporins could receive alternative treatments such as lincomamides, quinolones, and tetracyclines. The mortality rate in the TEN group was the highest at 20.0%, followed by the SJS/SJS-TEN overlap group (7.1%), and no deaths were observed in the DRESS and AGEP groups.
Conclusion: The identification of the culprit antibiotics and the application of alternative antibiotic therapies are crucial for the management of antibiotic-induced SCARs. If complicated underlying conditions and complications like advanced age, cancer and pneumonia coexist with SCARs, patients might be more at risk for mortality.
Keywords: AGEP; DRESS; SJS; TEN; antibiotic; retrospective study; severe cutaneous adverse reactions.
Copyright © 2024 Lu, Zhou, Zou, Wei, Zhou, Guo, Li, Ye and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Peter JG, Lehloenya R, Dlamini S, Risma K, White KD, Konvinse KC, et al. . Severe delayed cutaneous and systemic reactions to drugs: A global perspective on the science and art of current practice. J Allergy Clin Immunol Pract. (2017) 5(3):547–63. doi: 10.1016/j.jaip.2017.01.025 - DOI - PMC - PubMed
-
- Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. . ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. (2010) 88(1):60–8. doi: 10.1038/clpt.2009.252 - DOI - PubMed
-
- Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: A retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. (2008) 58(1):33–40. doi: 10.1016/j.jaad.2007.08.039 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

