Hepatic macrophage niche: a bridge between HBV-mediated metabolic changes with intrahepatic inflammation
- PMID: 39091506
- PMCID: PMC11291371
- DOI: 10.3389/fimmu.2024.1414594
Hepatic macrophage niche: a bridge between HBV-mediated metabolic changes with intrahepatic inflammation
Abstract
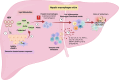
Hepatitis B Virus (HBV) is a stealthy and insidious pathogen capable of inducing chronic necro-inflammatory liver disease and hepatocellular carcinoma (HCC), resulting in over one million deaths worldwide per year. The traditional understanding of Chronic Hepatitis B (CHB) progression has focused on the complex interplay among ongoing virus replication, aberrant immune responses, and liver pathogenesis. However, the dynamic progression and crucial factors involved in the transition from HBV infection to immune activation and intrahepatic inflammation remain elusive. Recent insights have illuminated HBV's exploitation of the sodium taurocholate co-transporting polypeptide (NTCP) and manipulation of the cholesterol transport system shared between macrophages and hepatocytes for viral entry. These discoveries deepen our understanding of HBV as a virus that hijacks hepatocyte metabolism. Moreover, hepatic niche macrophages exhibit significant phenotypic and functional diversity, zonal characteristics, and play essential roles, either in maintaining liver homeostasis or contributing to the pathogenesis of chronic liver diseases. Therefore, we underscore recent revelations concerning the importance of hepatic niche macrophages in the context of viral hepatitis. This review particularly emphasizes the significant role of HBV-induced metabolic changes in hepatic macrophages as a key factor in the transition from viral infection to immune activation, ultimately culminating in liver inflammation. These metabolic alterations in hepatic macrophages offer promising targets for therapeutic interventions and serve as valuable early warning indicators, shedding light on the disease progression.
Keywords: HBV; hepatic macrophage niches; lipid metabolism (fatty acids); liver inflammation; metabolism.
Copyright © 2024 Wang, Lu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Charatcharoenwitthaya P, Pongpaibul A, Kaosombatwattana U, Bhanthumkomol P, Bandidniyamanon W, Pausawasdi N, et al. The prevalence of steatohepatitis in chronic hepatitis B patients and its impact on disease severity and treatment response. Liver Int. (2017) 37:542–51. doi: 10.1111/liv.13271 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

