CytoCellDB: a comprehensive resource for exploring extrachromosomal DNA in cancer cell lines
- PMID: 39091515
- PMCID: PMC11292414
- DOI: 10.1093/narcan/zcae035
CytoCellDB: a comprehensive resource for exploring extrachromosomal DNA in cancer cell lines
Abstract
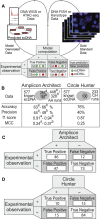
Recently, the cancer community has gained a heightened awareness of the roles of extrachromosomal DNA (ecDNA) in cancer proliferation, drug resistance and epigenetic remodeling. However, a hindrance to studying ecDNA is the lack of available cancer model systems that express ecDNA. Increasing our awareness of which model systems express ecDNA will advance our understanding of fundamental ecDNA biology and unlock a wealth of potential targeting strategies for ecDNA-driven cancers. To bridge this gap, we created CytoCellDB, a resource that provides karyotype annotations for cell lines within the Cancer Dependency Map (DepMap) and the Cancer Cell Line Encyclopedia (CCLE). We identify 139 cell lines that express ecDNA, a 200% increase from what is currently known. We expanded the total number of cancer cell lines with ecDNA annotations to 577, which is a 400% increase, covering 31% of cell lines in CCLE/DepMap. We experimentally validate several cell lines that we predict express ecDNA or homogeneous staining regions (HSRs). We demonstrate that CytoCellDB can be used to characterize aneuploidy alongside other molecular phenotypes, (gene essentialities, drug sensitivities, gene expression). We anticipate that CytoCellDB will advance cytogenomics research as well as provide insights into strategies for developing therapeutics that overcome ecDNA-driven drug resistance.
© The Author(s) 2024. Published by Oxford University Press on behalf of NAR Cancer.
Figures






References
-
- Pongor L.S., Schultz C.W., Rinaldi L., Wangsa D., Redon C.E., Takahashi N., Fialkoff G., Desai P., Zhang Y., Burkett S. et al. Extrachromosomal DNA amplification contributes to small cell lung cancer heterogeneity and is associated with worse outcomes. Cancer Discov. 2023; 13:928–949. - PMC - PubMed
-
- deCarvalho A.C., Kim H., Poisson L.M., Winn M.E., Mueller C., Cherba D., Koeman J., Seth S., Protopopov A., Felicella M. et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat. Genet. 2018; 50:708–717. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

