Brief Report: Real-World Eligibility for Clinical Trials in Patients With Extensive-Stage SCLC at a Tertiary Care Center
- PMID: 39091596
- PMCID: PMC11293570
- DOI: 10.1016/j.jtocrr.2024.100696
Brief Report: Real-World Eligibility for Clinical Trials in Patients With Extensive-Stage SCLC at a Tertiary Care Center
Abstract
Introduction: The CASPIAN and IMpower133 trials revealed a significant survival benefit of chemotherapy plus immunotherapy in patients with extensive-stage SCLC. The current study characterizes the proportion of real-world patients who would have met eligibility for these trials and highlights factors influencing eligibility in the real-world setting.
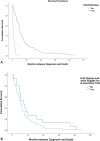
Methods: A retrospective analysis of patient data was conducted for stage IV patients with SCLC treated at the Cancer Centre of Southeastern Ontario, Canada. Trial eligibility was based on criteria used in the IMpower133 and CASPIAN trials. Data were summarized using descriptive statistics. Overall survival was assessed using the Kaplan-Meier method.
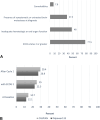
Results: Of the 116 patients included, only 12.1% met the overall eligibility criteria for the IMpower133 trial, and 14.7% for the CASPIAN trial. The most common reasons for ineligibility included: Eastern Cooperative Oncology Group (ECOG) 2 or greater (77.5%), inadequate organ function (48%), and the presence of brain metastases at diagnosis (37.3%). Sixty-one patients (59.8%) met two or more major ineligibility criteria. If trial eligibility was expanded to include ECOG 2 patients, an additional 10.3% would have met eligibility. The median overall survival for all-comers was 6.5 months.
Conclusions: Only a small minority of real-world patients with extensive-stage SCLC would have met eligibility for the IMpower133 and CASPIAN trials, with ECOG greater than or equal to 2, inadequate organ function, and brain metastases comprising the most common reasons for trial ineligibility. Future clinical trials should expand the inclusion criteria to better represent real-world patient populations.
Keywords: CASPIAN; Clinical trial eligibility; IMpower133; Real world; SCLC.
© 2024 The Authors.
Conflict of interest statement
Dr. Gaudreau has received travel support from the Canadian Cancer Trials Group, Canada (CCTG) to attend the CCTG Spring Meeting 2023, ASCO 2023 Annual Meeting, and the World Conference on Lung Cancer 2023 Annual Meeting. They have received grants from 10.13039/501100000015Canadian Cancer Society Research Institute: CCTG core funding, US NIH: Canadian Collaborating Clinical Trials Network – CCTG core funding, Cancer Research Institute: BR.36 and IND.240 CCTG trials funding, UHN: IND.236 and IND.239 CCTG trials -Stand Up To Cancer (SU2C) Canada – Canadian Cancer Society Breast Cancer Dream Team Research Funding: IND.237 CCTG trial. Novartis: IND.242 CCTG trial, BioAtla: IND.240 CCTG trial and AstraZeneca: IND.238, IND.239, IND.240 CCTG trials. All payments were made directly to the institution (CCTG). Dr. Fung has received Institutional research funding from 10.13039/100004325AstraZeneca. Dr. Robinson has received consulting fees from Merck Sharpe Dohme, AstraZeneca, and Bristol-Myers Squibb. The remaining authors declare no conflict of interest.
Figures
References
-
- Horn L., Mansfield A.S., Szczęsna A., et al. IMpower133 study group First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. - PubMed
-
- Paz-Ares L., Dvorkin M., Chen Y., et al. Caspian investigators Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. - PubMed
-
- Goldman J.W., Dvorkin M., Chen Y., et al. CASPIAN investigators Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22:51–65. - PubMed
-
- Harvey R.D., Bruinooge S.S., Chen L., et al. Impact of broadening trial eligibility criteria for patients with advanced non-small cell lung cancer: real-world analysis of select ASCO-friends recommendations. Clin Cancer Res. 2021;27:2430–2434. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

