Efficacy and safety of telitacicept in patients with lupus nephritis
- PMID: 39091632
- PMCID: PMC11292174
- DOI: 10.3892/etm.2024.12660
Efficacy and safety of telitacicept in patients with lupus nephritis
Abstract
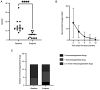
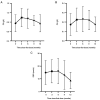
Although telitacicept is a promising drug for treating systemic lupus erythematosus, there are limited studies on its efficacy and safety in patients with lupus nephritis in China. This lack of research data restricts its potential for broader application and acceptance on a global scale. The present study aimed to determine the efficacy and safety of telitacicept in patients with lupus nephritis (LN) in China. Using a self-controlled before-after comparison method, patients with LN were recruited at Lishui Central Hospital between February 2022 and April 2023, who received telitacicept weekly as part of the standard treatment. Data on the systemic lupus erythematosus disease activity index 2000 (SLEDAI-2K), glucocorticoid dosing and the quantity of immunosuppressive medicines prescribed was collected. Additionally, serum complements, erythrocyte sedimentation rate (ESR), urinary protein levels, immunoglobulin concentrations, serum creatinine levels, plasma albumin concentrations, platelet counts and renal function parameters were documented throughout the study. A total of 13 patients were enrolled in the trial, comprising 11 women and two men. Following 12-48 weeks of treatment with telitacicept (80 or 160 mg per week), 84.6% (n=11) of all patients experienced symptom relief and their SLEDAI-2K score was reduced by more than four points. By the observation endpoint, the median glucocorticoid dosage of the 13 patients was decreased from 15 to 2.5 mg/d, and six patients discontinued their glucocorticoids. Furthermore, 46.1% of patients (n=6) reduced their dose and number of immunosuppressive medicines, while 15.4% (n=2) stopped their immunosuppressive medicines. Minimal changes were observed in serum creatinine, platelet count, C3 levels and C4 levels among patients. Immunoglobulin levels (IgG, IgA and IgM) remained stable or showed an upward trend. Plasma albumin levels remained within the normal range in three patients and increased in ten patients. It increased to the normal range in three of these ten patients. At the endpoint, ESR levels decreased in all patients. Additionally, three patients displayed varying degrees of renal function improvement, and their estimated glomerular filtration rate (ml/min/l.73 m2) increased from 127.8 to 134.2, 95.1 to 123.1 and 61.5 to 67.3, respectively. Urinary protein levels decreased in all patients. It decreased >0.5 g/l in seven patients and reached the normal levels in three patients. The adverse events of telitacicept were manageable. Among the patients infected with COVID-19, three patients had fever, 10 patients remained asymptomatic and none of them exhibited severe respiratory syndromes. In this study, telitacicept effectively stabilized LN activity and alleviated the clinical symptoms of most patients. Furthermore, it reduced the dose of glucocorticoid and immunosuppressive medicines. Therefore, telitacicept may be a promising treatment option for individuals with lupus nephritis.
Keywords: efficacy; lupus nephritis; safety; systemic lupus erythematosus; telitacicept.
Copyright: © 2024 Zhu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous
