Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease
- PMID: 39091710
- PMCID: PMC11290391
- DOI: 10.3748/wjg.v30.i28.3428
Effects of elafibranor on liver fibrosis and gut barrier function in a mouse model of alcohol-associated liver disease
Abstract
Background: Alcohol-associated liver disease (ALD) is a leading cause of liver-related morbidity and mortality, but there are no therapeutic targets and modalities to prevent ALD-related liver fibrosis. Peroxisome proliferator activated receptor (PPAR) α and δ play a key role in lipid metabolism and intestinal barrier homeostasis, which are major contributors to the pathological progression of ALD. Meanwhile, elafibranor (EFN), which is a dual PPARα and PPARδ agonist, has reached a phase III clinical trial for the treatment of metabolic dysfunction-associated steatotic liver disease and primary biliary cholangitis. However, the benefits of EFN for ALD treatment is unknown.
Aim: To evaluate the inhibitory effects of EFN on liver fibrosis and gut-intestinal barrier dysfunction in an ALD mouse model.
Methods: ALD-related liver fibrosis was induced in female C57BL/6J mice by feeding a 2.5% ethanol (EtOH)-containing Lieber-DeCarli liquid diet and intraperitoneally injecting carbon tetrachloride thrice weekly (1 mL/kg) for 8 weeks. EFN (3 and 10 mg/kg/day) was orally administered during the experimental period. Histological and molecular analyses were performed to assess the effect of EFN on steatohepatitis, fibrosis, and intestinal barrier integrity. The EFN effects on HepG2 lipotoxicity and Caco-2 barrier function were evaluated by cell-based assays.
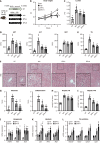
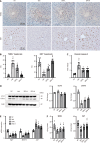
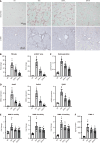
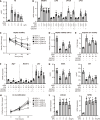
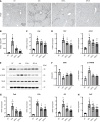
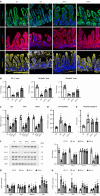
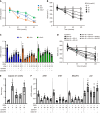
Results: The hepatic steatosis, apoptosis, and fibrosis in the ALD mice model were significantly attenuated by EFN treatment. EFN promoted lipolysis and β-oxidation and enhanced autophagic and antioxidant capacities in EtOH-stimulated HepG2 cells, primarily through PPARα activation. Moreover, EFN inhibited the Kupffer cell-mediated inflammatory response, with blunted hepatic exposure to lipopolysaccharide (LPS) and toll like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) signaling. EFN improved intestinal hyperpermeability by restoring tight junction proteins and autophagy and by inhibiting apoptosis and proinflammatory responses. The protective effect on intestinal barrier function in the EtOH-stimulated Caco-2 cells was predominantly mediated by PPARδ activation.
Conclusion: EFN reduced ALD-related fibrosis by inhibiting lipid accumulation and apoptosis, enhancing hepatocyte autophagic and antioxidant capacities, and suppressing LPS/TLR4/NF-κB-mediated inflammatory responses by restoring intestinal barrier function.
Keywords: Apoptosis; Autophagy; Ethanol; Gut barrier function; Liver fibrosis; Peroxisome proliferator activated receptor.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures
References
-
- Julien J, Ayer T, Bethea ED, Tapper EB, Chhatwal J. Projected prevalence and mortality associated with alcohol-related liver disease in the USA, 2019-40: a modelling study. Lancet Public Health. 2020;5:e316–e323. - PubMed
-
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516–537. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

