This is a preprint.
γ-aminobutyric acid receptor B signaling drives glioblastoma in females in an immune-dependent manner
- PMID: 39091833
- PMCID: PMC11291093
- DOI: 10.1101/2024.07.18.603996
γ-aminobutyric acid receptor B signaling drives glioblastoma in females in an immune-dependent manner
Abstract
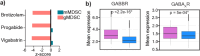
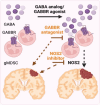
Sex differences in immune responses impact cancer outcomes and treatment response, including in glioblastoma (GBM). However, host factors underlying sex specific immune-cancer interactions are poorly understood. Here, we identify the neurotransmitter γ-aminobutyric acid (GABA) as a driver of GBM-promoting immune response in females. We demonstrated that GABA receptor B (GABBR) signaling enhances L-Arginine metabolism and nitric oxide synthase 2 (NOS2) expression in female granulocytic myeloid-derived suppressor cells (gMDSCs). GABBR agonist and GABA analog promoted GBM growth in females in an immune-dependent manner, while GABBR inhibition reduces gMDSC NOS2 production and extends survival only in females. Furthermore, female GBM patients have enriched GABA transcriptional signatures compared to males, and the use of GABA analogs in GBM patients is associated with worse short-term outcomes only in females. Collectively, these results highlight that GABA modulates anti-tumor immune response in a sex-specific manner, supporting future assessment of GABA pathway inhibitors as part of immunotherapy approaches.
Keywords: GABA; L-arginine; glioblastoma; myeloid-derived suppressor cells; nitric oxide synthase 2; sex differences.
Conflict of interest statement
Conflict of interest: In the past three years, C.A.L. has consulted for Astellas Pharmaceuticals, Odyssey Therapeutics, Third Rock Ventures, and T-Knife Therapeutics, and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-ME1 pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015). J.D.L reports being named as a co-inventor on pending and issued patents held by the Cleveland Clinic relating to cancer therapies, but these are not directly relevant to this work.
Figures








References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
