This is a preprint.
Endothelial TRPV4/Cx43 Signaling Complex Regulates Vasomotor Tone in Resistance Arteries
- PMID: 39091840
- PMCID: PMC11291137
- DOI: 10.1101/2024.07.25.604930
Endothelial TRPV4/Cx43 Signaling Complex Regulates Vasomotor Tone in Resistance Arteries
Update in
-
Endothelial TRPV4-Cx43 signalling complex regulates vasomotor tone in resistance arteries.J Physiol. 2025 Aug;603(15):4345-4366. doi: 10.1113/JP285194. Epub 2025 Feb 21. J Physiol. 2025. PMID: 39982706 Free PMC article.
Abstract
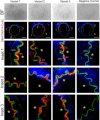
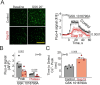
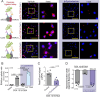
S-nitrosylation of Cx43 gap junction channels critically regulates communication between smooth muscle cells and endothelial cells. This posttranslational modification also induces the opening of undocked Cx43 hemichannels. However, its specific impact on vasomotor regulation remains unclear. Considering the role of endothelial TRPV4 channel activation in promoting vasodilation through nitric oxide (NO) production, we investigated the direct modulation of endothelial Cx43 hemichannels by TRPV4 channel activation. Using the proximity ligation assay, we identify that Cx43 and TRPV4 are found in close proximity in the endothelium of resistance arteries. In primary endothelial cell cultures from resistance arteries (ECs), GSK-induced TRPV4 activation enhances eNOS activity, increases NO production, and opens Cx43 hemichannels via direct S-nitrosylation. Notably, the elevated intracellular Ca2+ levels caused by TRPV4 activation were reduced by blocking Cx43 hemichannels. In ex vivo mesenteric arteries, inhibiting Cx43 hemichannels reduced endothelial hyperpolarization without affecting NO production in ECs, underscoring a critical role of TRPV4/Cx43 signaling in endothelial electrical behavior. We perturbed the proximity of Cx43/TRPV4 by disrupting lipid rafts in ECs using β-cyclodextrin. Under these conditions, hemichannel activity, Ca2+ influx, and endothelial hyperpolarization were blunted upon GSK stimulation. Intravital microscopy of mesenteric arterioles in vivo further demonstrated that inhibiting Cx43 hemichannels activity, NO production and disrupting endothelial integrity reduce TRPV4-induced relaxation. These findings underscore a new pivotal role of Cx43 hemichannel associated with TRPV4 signaling pathway in modulating endothelial electrical behavior and vasomotor tone regulation.
Keywords: Caveolae; ECs; hyperpolarization; resting membrane potential.
Figures

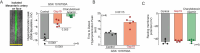

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
