This is a preprint.
Chemokines Kill Bacteria by Binding Anionic Phospholipids without Triggering Antimicrobial Resistance
- PMID: 39091850
- PMCID: PMC11291121
- DOI: 10.1101/2024.07.25.604863
Chemokines Kill Bacteria by Binding Anionic Phospholipids without Triggering Antimicrobial Resistance
Update in
-
Chemokines kill bacteria without triggering antimicrobial resistance by binding anionic phospholipids.Sci Adv. 2025 Jun 6;11(23):eads2675. doi: 10.1126/sciadv.ads2675. Epub 2025 Jun 6. Sci Adv. 2025. PMID: 40479071 Free PMC article.
Abstract
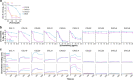
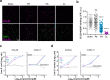
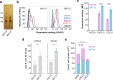
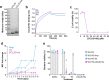
Classically, chemokines coordinate leukocyte trafficking during immune responses; however, many chemokines have also been reported to possess direct antibacterial activity in vitro. Yet, the bacterial killing mechanism of chemokines and the biochemical properties that define which members of the chemokine superfamily are antimicrobial remain poorly understood. Here we report that the antimicrobial activity of chemokines is defined by their ability to bind phosphatidylglycerol and cardiolipin, two anionic phospholipids commonly found in the bacterial plasma membrane. We show that only chemokines able to bind these two phospholipids kill Escherichia coli and Staphylococcus aureus and that they exert rapid bacteriostatic and bactericidal effects against E. coli with a higher potency than the antimicrobial peptide beta-defensin 3. Furthermore, our data support that bacterial membrane cardiolipin facilitates the antimicrobial action of chemokines. Both biochemical and genetic interference with the chemokine-cardiolipin interaction impaired microbial growth arrest, bacterial killing, and membrane disruption by chemokines. Moreover, unlike conventional antibiotics, E. coli failed to develop resistance when placed under increasing antimicrobial chemokine pressure in vitro. Thus, we have identified cardiolipin and phosphatidylglycerol as novel binding partners for chemokines responsible for chemokine antimicrobial action. Our results provide proof of principle for developing chemokines as novel antibiotics resistant to bacterial antimicrobial resistance mechanisms.
Keywords: Antimicrobial peptides; antibiotics; cardiolipin; multidrug-resistant microorganisms; phosphatidylglycerol; phospholipids.
Conflict of interest statement
CONFLICT OF INTEREST B.F.V has ownership interests in Protein Foundry, LLC and XLock Biosciences, Inc. All other authors declare no competing interests.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
