This is a preprint.
Parallel patterns of cognitive aging in marmosets and macaques
- PMID: 39091859
- PMCID: PMC11291085
- DOI: 10.1101/2024.07.22.604411
Parallel patterns of cognitive aging in marmosets and macaques
Update in
-
Parallel patterns of age-related working memory impairment in marmosets and macaques.Aging (Albany NY). 2025 Mar 24;17(3):778-797. doi: 10.18632/aging.206225. Epub 2025 Mar 24. Aging (Albany NY). 2025. PMID: 40131878 Free PMC article.
Abstract
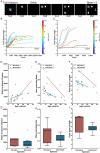
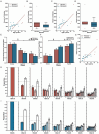
As humans age, some experience cognitive impairment while others do not. When impairment does occur, it is not expressed uniformly across cognitive domains and varies in severity across individuals. Translationally relevant model systems are critical for understanding the neurobiological drivers of this variability, which is essential to uncovering the mechanisms underlying the brain's susceptibility to the effects of aging. As such, non-human primates are particularly important due to shared behavioral, neuroanatomical, and age-related neuropathological features with humans. For many decades, macaque monkeys have served as the primary non-human primate model for studying the neurobiology of cognitive aging. More recently, the common marmoset has emerged as an advantageous model for this work due to its short lifespan that facilitates longitudinal studies. Despite their growing popularity as a model, whether marmosets exhibit patterns of age-related cognitive impairment comparable to those observed in macaques and humans remains unexplored. To address this major limitation for the development and evaluation of the marmoset as a model of cognitive aging, we directly compared working memory ability as a function of age in macaques and marmosets on the identical working memory task. Our results demonstrate that marmosets and macaques exhibit remarkably similar age-related working memory deficits, highlighting the value of the marmoset as a model for cognitive aging research within the neuroscience community.
Keywords: aging; cognitive impairment; comparative cognition; macaque; marmoset; monkey; non-human primate; working memory.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB (1999) Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus 9:562–574. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
