Decreased Circulating Very Small Low-Density Lipoprotein is Likely Causal for Age-Related Macular Degeneration
- PMID: 39091897
- PMCID: PMC11292535
- DOI: 10.1016/j.xops.2024.100535
Decreased Circulating Very Small Low-Density Lipoprotein is Likely Causal for Age-Related Macular Degeneration
Abstract
Objective: Abnormal changes in metabolite levels in serum or plasma have been highlighted in several studies in age-related macular degeneration (AMD), the leading cause of irreversible vision loss. Specific changes in lipid profiles are associated with an increased risk of AMD. Metabolites could thus be used to investigate AMD disease mechanisms or incorporated into AMD risk prediction models. However, whether particular metabolites causally affect the disease has yet to be established.
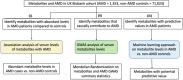
Design: A 3-tiered analysis of blood metabolites in the United Kingdom (UK) Biobank cohort to identify metabolites that differ in AMD patients with evidence for a putatively causal role in AMD.
Participants: A total of 72 376 donors from the UK Biobank cohort including participants with AMD (N = 1353) and non-AMD controls (N = 71 023).
Methods: We analyzed 325 directly measured or derived blood metabolites from the UK Biobank for 72 376 donors to identify AMD-associated metabolites. Genome-wide association studies for 325 metabolites in 98 316 European participants from the UK Biobank were performed. The causal effects of these metabolites in AMD were tested using a 2-sample Mendelian randomization approach. The predictive value of these measurements together with sex and age was assessed by developing a machine learning classifier.
Main outcome measures: Evaluating metabolic biomarkers associated with AMD susceptibility and investigating their potential causal contribution to the development of the disease.
Results: This study noted age to be the prominent risk factor associated with AMD development. While accounting for age and sex, we identified 84 metabolic markers as significantly (false discovery rate-adjusted P value < 0.05) associated with AMD. Lipoprotein subclasses comprised the majority of the AMD-associated metabolites (39%) followed by several lipoprotein to lipid ratios. Nineteen metabolites showed a likely causative role in AMD etiology. Of these, 6 lipoproteins contain very small, very low-density lipoprotein (VLDL), and phospholipids to total lipid ratio in medium VLDL. Based on this we postulate that depletion of circulating very small VLDLs is likely causal for AMD. The risk prediction model constructed from the metabolites, age and sex, identified age as the primary predictive factor with a much smaller contribution by metabolites to AMD risk prediction.
Conclusions: This study underscores the pronounced role of lipids in AMD susceptibility and the likely causal contribution of particular subclasses of lipoproteins to AMD. Our study provides valuable insights into the metabopathological mechanisms of AMD disease development and progression.
Keywords: Age-related macular degeneration; Circulating metabolites; Genome-wide association studies; Mendelian randomization.
Crown Copyright © 2024 Published by Elsevier Inc. on behalf of the American Academy of Ophthalmology.
Figures



References
LinkOut - more resources
Full Text Sources

