3D bioprinted CRC model brings to light the replication necessity of an oncolytic vaccinia virus encoding FCU1 gene to exert an efficient anti-tumoral activity
- PMID: 39091906
- PMCID: PMC11292208
- DOI: 10.3389/fonc.2024.1384499
3D bioprinted CRC model brings to light the replication necessity of an oncolytic vaccinia virus encoding FCU1 gene to exert an efficient anti-tumoral activity
Abstract
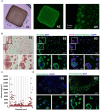
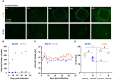
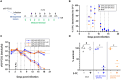
The oncolytic virus represents a promising therapeutic strategy involving the targeted replication of viruses to eliminate cancer cells, while preserving healthy ones. Despite ongoing clinical trials, this approach encounters significant challenges. This study delves into the interaction between an oncolytic virus and extracellular matrix mimics (ECM mimics). A three-dimensional colorectal cancer model, enriched with ECM mimics through bioprinting, was subjected to infection by an oncolytic virus derived from the vaccinia virus (oVV). The investigation revealed prolonged expression and sustained oVV production. However, the absence of a significant antitumor effect suggested that the virus's progression toward non-infected tumoral clusters was hindered by the ECM mimics. Effective elimination of tumoral cells was achieved by introducing an oVV expressing FCU1 (an enzyme converting the prodrug 5-FC into the chemotherapeutic compound 5-FU) alongside 5-FC. Notably, this efficacy was absent when using a non-replicative vaccinia virus expressing FCU1. Our findings underscore then the crucial role of oVV proliferation in a complex ECM mimics. Its proliferation facilitates payload expression and generates a bystander effect to eradicate tumors. Additionally, this study emphasizes the utility of 3D bioprinting for assessing ECM mimics impact on oVV and demonstrates how enhancing oVV capabilities allows overcoming these barriers. This showcases the potential of 3D bioprinting technology in designing purpose-fit models for such investigations.
Keywords: bioprinting; colorectal (colon) cancer; hydrogel; oncovirus; tumor.
Copyright © 2024 Marquette, Petiot, Spindler, Ebel, Nzepa, Moreau, Erbs, Balloul, Quemeneur and Zaupa.
Conflict of interest statement
Author AS, CE, MN, BM, PE, J-MB, EQ and CZ were employed by company Transgene SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

