Bile acids attenuate hepatic inflammation during ischemia/reperfusion injury
- PMID: 39091991
- PMCID: PMC11292370
- DOI: 10.1016/j.jhepr.2024.101101
Bile acids attenuate hepatic inflammation during ischemia/reperfusion injury
Abstract
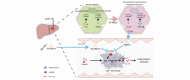
Background & aims: Persistent cholestasis has been associated with poor prognosis after orthotopic liver transplantation. In this study, we aimed to investigate how the accumulation of tauro-beta-muricholic acid (TβMCA), resulting from the reprogramming of bile acid (BA) metabolism during liver ischemia/reperfusion (IR) stress, attenuates liver inflammation.
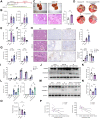
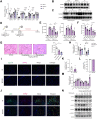
Methods: Ingenuity Pathway Analysis was performed using transcriptome data from a murine hepatic IR model. Three different models of hepatic IR (liver warm IR, bile duct separation-IR, common bile duct ligation-IR) were employed. We generated adeno-associated virus-transfected mice and CD11b-DTR mice to assess the role of BAs in regulating the myeloid S1PR2-GSDMD axis. Hepatic BA levels were analyzed using targeted metabolomics. Finally, the correlation between the reprogramming of BA metabolism and hepatic S1PR2 levels was validated through RNA-seq of human liver transplant biopsies.
Results: We found that BA metabolism underwent reprogramming in murine hepatocytes under IR stress, leading to increased synthesis of TβMCA, catalyzed by the enzyme CYP2C70. The levels of hepatic TβMCA were negatively correlated with the severity of hepatic inflammation, as indicated by the serum IL-1β levels. Inhibition of hepatic CYP2C70 resulted in reduced TβMCA production, which subsequently increased serum IL-1β levels and exacerbated IR injury. Moreover, our findings suggested that TβMCA could inhibit canonical inflammasome activation in macrophages and attenuate inflammatory responses in a myeloid-specific S1PR2-GSDMD-dependent manner. Additionally, Gly-βMCA, a derivative of TβMCA, could effectively attenuate inflammatory injury in vivo and inhibit human macrophage pyroptosis in vitro.
Conclusions: IR stress orchestrates hepatic BA metabolism to generate TβMCA, which attenuates hepatic inflammatory injury by inhibiting the myeloid S1PR2-GSDMD axis. Bile acids have immunomodulatory functions in liver reperfusion injury that may guide therapeutic strategies.
Impact and implications: Our research reveals that liver ischemia-reperfusion stress triggers reprogramming of bile acid metabolism. This functions as an adaptive mechanism to mitigate inflammatory injury by regulating the S1PR2-GSDMD axis, thereby controlling the release of IL-1β from macrophages. Our results highlight the crucial role of bile acids in regulating hepatocyte-immune cell crosstalk, which demonstrates an immunomodulatory function in liver reperfusion injury that may guide therapeutic strategies targeting bile acids and their receptors.
Keywords: CYP2C70; GSDMD; Liver transplantation; Macrophages; Pyroptosis; S1PR2.
© 2024 The Authors.
Figures





References
-
- Hirao H., Nakamura K., Kupiec-Weglinski J.W. Liver ischaemia-reperfusion injury: a new understanding of the role of innate immunity. Nat Rev Gastroenterol Hepatol. 2022;19:239–256. - PubMed
-
- Bernhardt G.A., Zollner G., Cerwenka H., et al. Hepatobiliary transporter expression and post-operative jaundice in patients undergoing partial hepatectomy. Liver Int. 2012;32:119–127. - PubMed
-
- Buis C.I., Geuken E., Visser D.S., et al. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol. 2009;50:69–79. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

