Drosophila larval motor patterning relies on regulated alternative splicing of Dscam2
- PMID: 39092203
- PMCID: PMC11292952
- DOI: 10.3389/fnmol.2024.1415207
Drosophila larval motor patterning relies on regulated alternative splicing of Dscam2
Abstract
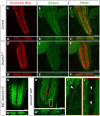
Recent studies capitalizing on the newly complete nanometer-resolution Drosophila larval connectome have made significant advances in identifying the structural basis of motor patterning. However, the molecular mechanisms utilized by neurons to wire these circuits remain poorly understood. In this study we explore how cell-specific expression of two Dscam2 isoforms, which mediate isoform-specific homophilic binding, contributes to motor patterning and output of Drosophila larvae. Ablating Dscam2 isoform diversity resulted in impaired locomotion. Electrophysiological assessment at the neuromuscular junction during fictive locomotion indicated that this behavioral defect was largely caused by weaker bouts of motor neuron activity. Morphological analyses of single motor neurons using MultiColour FlpOut revealed severe errors in dendrite arborization and assessment of cholinergic and GABAergic projections to the motor domain revealed altered morphology of interneuron processes. Loss of Dscam2 did not affect locomotor output, motor neuron activation or dendrite targeting. Our findings thus suggest that locomotor circuit phenotypes arise specifically from inappropriate Dscam2 interactions between premotor interneurons and motor neurons when they express the same isoform. Indeed, we report here that first-order premotor interneurons express Dscam2A. Since motor neurons express Dscam2B, our results provide evidence that Dscam2 isoform expression alternates between synaptic partners in the nerve cord. Our study demonstrates the importance of cell-specific alternative splicing in establishing the circuitry that underlies neuromotor patterning without inducing unwanted intercellular interactions.
Keywords: A02; Drosophila; Dscam2; alternative splicing; dendrite targeting; fictive locomotion; looper; motor neuron.
Copyright © 2024 Odierna, Kerwin, Shin and Millard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

