The osteocytic actions of glucocorticoids on bone mass, mechanical properties, or perilacunar remodeling outcomes are not rescued by PTH(1-34)
- PMID: 39092287
- PMCID: PMC11291448
- DOI: 10.3389/fendo.2024.1342938
The osteocytic actions of glucocorticoids on bone mass, mechanical properties, or perilacunar remodeling outcomes are not rescued by PTH(1-34)
Abstract
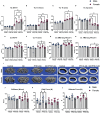
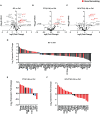
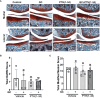
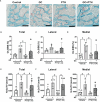
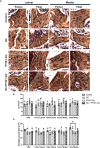
Glucocorticoids (GC) and parathyroid hormone (PTH) are widely used therapeutic endocrine hormones where their effects on bone and joint arise from actions on multiple skeletal cell types. In osteocytes, GC and PTH exert opposing effects on perilacunar canalicular remodeling (PLR). Suppressed PLR can impair bone quality and joint homeostasis, including in GC-induced osteonecrosis. However, combined effects of GC and PTH on PLR are unknown. Given the untapped potential to target osteocytes to improve skeletal health, this study sought to test the feasibility of therapeutically mitigating PLR suppression. Focusing on subchondral bone and joint homeostasis, we hypothesize that PTH(1-34), a PLR agonist, could rescue GC-suppressed PLR. The skeletal effects of GC and PTH(1-34), alone or combined, were examined in male and female mice by micro-computed tomography, mechanical testing, histology, and gene expression analysis. For each outcome, females were more responsive to GC and PTH(1-34) than males. GC and PTH(1-34) exerted regional differences, with GC increasing trabecular bone volume but reducing cortical bone thickness, stiffness, and ultimate force. Despite PTH(1-34)'s anabolic effects on trabecular bone, it did not rescue GC's catabolic effects on cortical bone. Likewise, cartilage integrity and subchondral bone apoptosis, tartrate-resistant acid phosphatase (TRAP) activity, and osteocyte lacunocanalicular networks showed no evidence that PTH(1-34) could offset GC-dependent effects. Rather, GC and PTH(1-34) each increased cortical bone gene expression implicated in bone resorption by osteoclasts and osteocytes, including Acp5, Mmp13, Atp6v0d2, Ctsk, differences maintained when GC and PTH(1-34) were combined. Since PTH(1-34) is insufficient to rescue GC's effects on young female mouse bone, future studies are needed to determine if osteocyte PLR suppression, due to GC, aging, or other factors, can be offset by a PLR agonist.
Keywords: PTH (1-34); bone; glucocorticoids; osteocyte; osteocytic osteolysis; parathyroid hormone (PTH); perilacunar canalicular remodeling; prednisolone.
Copyright © 2024 Yee, Meliadis, Kaya, Chang and Alliston.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Wiedmeier SE, Samlowski WE, Rasmussen CJ, Huang K, Daynes RA. Effect of ionizing radiation on thymic epithelial cell function. I. Radiation-spared thymic epithelial grafts expedite the recovery of T cell function in lethally irradiated and fetal liver reconstituted mice. J Immunol. (1988) 140:21–9. doi: 10.4049/jimmunol.140.1.21 - DOI - PubMed
-
- Yao W, Cheng Z, Pham A, Busse C, Zimmermann EA, Ritchie RO, et al. Glucocorticoid-induced bone loss in mice can be reversed by the actions of parathyroid hormone and risedronate on different pathways for bone formation and mineralization. Arthritis Rheum. (2008) 58:3485–97. doi: 10.1002/art.23954 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

