Potential association of SULT2A1 and ABCG2 variant alleles with increased risk for palbociclib toxicity
- PMID: 39092502
- PMCID: PMC11418216
- DOI: 10.1080/14622416.2024.2380240
Potential association of SULT2A1 and ABCG2 variant alleles with increased risk for palbociclib toxicity
Abstract
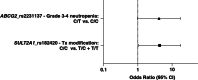
Aim: This study evaluated associations between CYP3A4*22 and variants in other pharmacogenes (CYP3A5, SULT2A1, ABCB1, ABCG2, ERCC1) and the risk for palbociclib-associated toxicities.Materials & methods: Two hundred cancer patients who received standard-of-care palbociclib were genotyped and associations with toxicity were evaluated retrospectively.Results: No significant associations were found for CYP3A4*22, CYP3A5*3, ABCB1_rs1045642, ABCG2_rs2231142, ERCC1_rs3212986 and ERCC1_rs11615. Homozygous variant carriers of SULT2A1_rs182420 had higher incidence of dose modifications due to palbociclib toxicity (odds ratio [OR]: 4.334, 95% CI: 1.057-17.767, p = 0.042). ABCG2_rs2231137 variant carriers had borderline higher incidence of grade 3-4 neutropenia (OR: 4.14, 95% CI: 0.99-17.37, p = 0.052).Conclusion: Once validated, SULT2A1 and ABCG2 variants may be useful to individualize palbociclib dosing to minimize toxicities and improve treatment outcomes.
Keywords: Breast cancer; CDK4/6 inhibitors; palbociclib; pharmacogenetics; toxicity.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
Similar articles
-
Genetic Variants in the ABCB1 and ABCG2 Gene Drug Transporters Involved in Gefitinib-Associated Adverse Reaction: A Systematic Review and Meta-Analysis.Genes (Basel). 2024 May 7;15(5):591. doi: 10.3390/genes15050591. Genes (Basel). 2024. PMID: 38790220 Free PMC article.
-
Evaluation of the Association of Polymorphisms With Palbociclib-Induced Neutropenia: Pharmacogenetic Analysis of PALOMA-2/-3.Oncologist. 2021 Jul;26(7):e1143-e1155. doi: 10.1002/onco.13811. Epub 2021 Jun 7. Oncologist. 2021. PMID: 33955129 Free PMC article.
-
Transporter Genes and statin-induced Hepatotoxicity.Cardiovasc Drugs Ther. 2025 Aug;39(4):801-810. doi: 10.1007/s10557-024-07580-2. Epub 2024 May 29. Cardiovasc Drugs Ther. 2025. PMID: 38809397
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Unveiling hidden risks: pharmacogenetic insights from a cross-sectional study of statin therapy in the Indian population.Pharmacol Rep. 2025 Aug;77(4):1040-1049. doi: 10.1007/s43440-025-00746-1. Epub 2025 Jun 9. Pharmacol Rep. 2025. PMID: 40489054
References
-
- NCCN clinical practice guidelines in oncology – breast cancer (version 2.2024). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. - PubMed
-
- Marineau A, St-Pierre C, Lessard-Hurtubise R, David ME, Adam JP, Chabot I. Cyclin-dependent kinase 4/6 inhibitor treatment use in women treated for advanced breast cancer: integrating ASCO/NCODA patient-centered standards in a community pharmacy. J Oncol Pharm Pract. 2023;29(5):1144–1153. doi: 10.1177/10781552221102884 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
