Geometric and dosimetric evaluation for breast and regional nodal auto-segmentation structures
- PMID: 39092893
- PMCID: PMC11466470
- DOI: 10.1002/acm2.14461
Geometric and dosimetric evaluation for breast and regional nodal auto-segmentation structures
Abstract
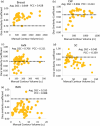
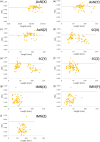
The accuracy of artificial intelligence (AI) generated contours for intact-breast and post-mastectomy radiotherapy plans was evaluated. Geometric and dosimetric comparisons were performed between auto-contours (ACs) and manual-contours (MCs) produced by physicians for target structures. Breast and regional nodal structures were manually delineated on 66 breast cancer patients. ACs were retrospectively generated. The characteristics of the breast/post-mastectomy chestwall (CW) and regional nodal structures (axillary [AxN], supraclavicular [SC], internal mammary [IM]) were geometrically evaluated by Dice similarity coefficient (DSC), mean surface distance, and Hausdorff Distance. The structures were also evaluated dosimetrically by superimposing the MC clinically delivered plans onto the ACs to assess the impact of utilizing ACs with target dose (Vx%) evaluation. Positive geometric correlations between volume and DSC for intact-breast, AxN, and CW were observed. Little or anti correlations between volume and DSC for IM and SC were shown. For intact-breast plans, insignificant dosimetric differences between ACs and MCs were observed for AxNV95% (p = 0.17) and SCV95% (p = 0.16), while IMNV90% ACs and MCs were significantly different. The average V95% for intact-breast MCs (98.4%) and ACs (97.1%) were comparable but statistically different (p = 0.02). For post-mastectomy plans, AxNV95% (p = 0.35) and SCV95% (p = 0.08) were consistent between ACs and MCs, while IMNV90% was significantly different. Additionally, 94.1% of AC-breasts met ΔV95% variation <5% when DSC > 0.7. However, only 62.5% AC-CWs achieved the same metrics, despite AC-CWV95% (p = 0.43) being statistically insignificant. The AC intact-breast structure was dosimetrically similar to MCs. The AC AxN and SC may require manual adjustments. Careful review should be performed for AC post-mastectomy CW and IMN before treatment planning. The findings of this study may guide the clinical decision-making process for the utilization of AI-driven ACs for intact-breast and post-mastectomy plans. Before clinical implementation of this auto-segmentation software, an in-depth assessment of agreement with each local facilities MCs is needed.
Keywords: AI‐algorithm; Auto‐contour; auto‐segmentation; breast radiation treatment.
© 2024 The Author(s). Journal of Applied Clinical Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
The authors have no affiliations with or involvement in any organization or entity with any financial interest.
Figures



Similar articles
-
Clinical feasibility of deep learning-based auto-segmentation of target volumes and organs-at-risk in breast cancer patients after breast-conserving surgery.Radiat Oncol. 2021 Feb 25;16(1):44. doi: 10.1186/s13014-021-01771-z. Radiat Oncol. 2021. PMID: 33632248 Free PMC article.
-
Evaluating the dosimetric impact of deep-learning-based auto-segmentation in prostate cancer radiotherapy: Insights into real-world clinical implementation and inter-observer variability.J Appl Clin Med Phys. 2025 Mar;26(3):e14569. doi: 10.1002/acm2.14569. Epub 2024 Dec 1. J Appl Clin Med Phys. 2025. PMID: 39616629 Free PMC article.
-
The dosimetric impact of deep learning-based auto-segmentation of organs at risk on nasopharyngeal and rectal cancer.Radiat Oncol. 2021 Jun 23;16(1):113. doi: 10.1186/s13014-021-01837-y. Radiat Oncol. 2021. PMID: 34162410 Free PMC article.
-
Geometric and dosimetric evaluations of atlas-based segmentation methods of MR images in the head and neck region.Phys Med Biol. 2018 Jul 11;63(14):145007. doi: 10.1088/1361-6560/aacb65. Phys Med Biol. 2018. PMID: 29882749 Free PMC article.
-
Evaluating the clinical acceptability of deep learning contours of prostate and organs-at-risk in an automated prostate treatment planning process.Med Phys. 2022 Apr;49(4):2570-2581. doi: 10.1002/mp.15525. Epub 2022 Feb 21. Med Phys. 2022. PMID: 35147216
Cited by
-
Automated segmentation of target volumes in breast cancer radiotherapy, impact on target size and dose to organs at risk.Clin Transl Radiat Oncol. 2025 May 28;53:100986. doi: 10.1016/j.ctro.2025.100986. eCollection 2025 Jul. Clin Transl Radiat Oncol. 2025. PMID: 40529410 Free PMC article.
-
Radiotherapy improves survival in HER2-positive breast cancer with lung metastases: a retrospective study with artificial intelligence-based prognostic modeling.Front Oncol. 2025 Jul 16;15:1633448. doi: 10.3389/fonc.2025.1633448. eCollection 2025. Front Oncol. 2025. PMID: 40740870 Free PMC article.
-
Clinical feasibility of Ethos auto-segmentation for adaptive whole-breast cancer treatment.Front Oncol. 2024 Dec 10;14:1507806. doi: 10.3389/fonc.2024.1507806. eCollection 2024. Front Oncol. 2024. PMID: 39720564 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

