Lacticaseibacillus rhamnosus HA-114 and Bacillus subtilis R0179 Prolong Lifespan and Mitigate Amyloid-β Toxicity in C. elegans via Distinct Mechanisms
- PMID: 39093068
- PMCID: PMC11380293
- DOI: 10.3233/JAD-230948
Lacticaseibacillus rhamnosus HA-114 and Bacillus subtilis R0179 Prolong Lifespan and Mitigate Amyloid-β Toxicity in C. elegans via Distinct Mechanisms
Abstract
Background: Recent advances linking gut dysbiosis with neurocognitive disorders such as Alzheimer's disease (AD) suggest that the microbiota-gut-brain axis could be targeted for AD prevention, management, or treatment.
Objective: We sought to identify probiotics that can delay Aβ-induced paralysis.
Methods: Using C. elegans expressing human amyloid-β (Aβ)1-42 in body wall muscles (GMC101), we assessed the effects of several probiotic strains on paralysis.
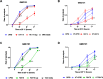
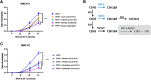
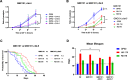
Results: We found that Lacticaseibacillus rhamnosus HA-114 and Bacillus subtilis R0179, but not their supernatants or heat-treated forms, delayed paralysis and prolonged lifespan without affecting the levels of amyloid-β aggregates. To uncover the mechanism involved, we explored the role of two known pathways involved in neurogenerative diseases, namely mitophagy, via deletion of the mitophagy factor PINK-1, and fatty acid desaturation, via deletion of the Δ9 desaturase FAT-5. Pink-1 deletion in GMC101 worms did not modify the life-prolonging and anti-paralysis effects of HA-114 but reduced the protective effect of R0179 against paralysis without affecting its life-prolonging effect. Upon fat5 deletion in GMC101 worms, the monounsaturated C14:1 and C16:1 FAs conserved their beneficial effect while the saturated C14:0 and C16:0 FAs did not. The beneficial effects of R0179 on both lifespan and paralysis remained unaffected by fat-5 deletion, while the beneficial effect of HA-114 on paralysis and lifespan was significantly reduced.
Conclusions: Collectively with clinical and preclinical evidence in other models, our results suggest that HA-114 or R0179 could be studied as potential therapeutical adjuncts in neurodegenerative diseases such as AD.
Keywords: Age-related diseases; Alzheimer’s disease; B. subtilis R0179; Caenorhabditis elegans; L. rhamnosus HA-114; amyloid; lipid metabolism; probiotics.
Conflict of interest statement
Stuart G. Foster, Shibi Mathew, Thomas A. Tompkins, and Sylvie Binda are employed by Lallemand Health Solutions Inc., a company that manufactures and markets the tested probiotics to business clients but not to consumers. All other authors have no conflict of interest to report.
Figures



Similar articles
-
Bacillus Subtilis Delays Neurodegeneration and Behavioral Impairment in the Alzheimer's Disease Model Caenorhabditis Elegans.J Alzheimers Dis. 2020;73(3):1035-1052. doi: 10.3233/JAD-190837. J Alzheimers Dis. 2020. PMID: 31884470
-
Monascin from Monascus-Fermented Products Reduces Oxidative Stress and Amyloid-β Toxicity via DAF-16/FOXO in Caenorhabditis elegans.J Agric Food Chem. 2016 Sep 28;64(38):7114-20. doi: 10.1021/acs.jafc.6b02779. Epub 2016 Sep 14. J Agric Food Chem. 2016. PMID: 27554775
-
rBTI reduced β-amyloid-induced toxicity by promoting autophagy-lysosomal degradation via DAF-16 in Caenorhabditis elegans.Exp Gerontol. 2017 Mar;89:78-86. doi: 10.1016/j.exger.2017.01.018. Epub 2017 Jan 22. Exp Gerontol. 2017. PMID: 28119052
-
Amyloid-beta (Aβ1-42)-induced paralysis in Caenorhabditis elegans is reduced by restricted cholesterol supply.Neurosci Lett. 2014 Jul 25;576:93-6. doi: 10.1016/j.neulet.2014.05.059. Epub 2014 Jun 5. Neurosci Lett. 2014. PMID: 24909620
-
Commensals, probiotics and pathogens in the Caenorhabditis elegans model.Cell Microbiol. 2014 Jan;16(1):27-38. doi: 10.1111/cmi.12234. Epub 2013 Nov 15. Cell Microbiol. 2014. PMID: 24168639 Review.
Cited by
-
Probiotic attributes, antioxidant and neuromodulatory effects of GABA-Producing Lactiplantibacillus plantarum SY1 and optimization of GABA production.BMC Microbiol. 2025 May 29;25(1):341. doi: 10.1186/s12866-025-04070-9. BMC Microbiol. 2025. PMID: 40442580 Free PMC article.
-
Probiotics as Potential Treatments for Neurodegenerative Diseases: a Review of the Evidence from in vivo to Clinical Trial.Biomol Ther (Seoul). 2025 Jan 1;33(1):54-74. doi: 10.4062/biomolther.2024.215. Epub 2024 Dec 16. Biomol Ther (Seoul). 2025. PMID: 39676295 Free PMC article. Review.
References
-
- Chakraborty A and Diwan A. Alzheimer and it’s possible therapy: a review. J Exp Neurol 2020; 1(4): 115–122.
-
- World Health Organization (2020) Dementia. https://www.who.int/news-room/fact-sheets/detail/dementia (2020, accessed 22 July 2021).
-
- Madav Y, Wairkar S and Prabhakar B. Recent therapeutic strategies targeting beta amyloid and tauopathies in Alzheimer’s disease. Brain Res Bull 2019; 146: 171–184. - PubMed
-
- Bennett DA. Lack of benefit with idalopirdine for Alzheimer disease: Another therapeutic failure in a complex disease process. JAMA 2018; 319: 123–125. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

