Real-world efficacy of a teriparatide biosimilar (RGB-10) compared with reference teriparatide on bone mineral density, trabecular bone score, and bone parameters assessed using quantitative ultrasound, 3D-SHAPER® and high-resolution peripheral computer tomography in postmenopausal women with osteoporosis and very high fracture risk
- PMID: 39093439
- PMCID: PMC11579072
- DOI: 10.1007/s00198-024-07208-z
Real-world efficacy of a teriparatide biosimilar (RGB-10) compared with reference teriparatide on bone mineral density, trabecular bone score, and bone parameters assessed using quantitative ultrasound, 3D-SHAPER® and high-resolution peripheral computer tomography in postmenopausal women with osteoporosis and very high fracture risk
Abstract
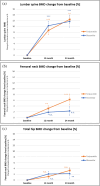
A retrospective analysis comparing a teriparatide biosimilar (RGB-10) with reference teriparatide for osteoporosis treatment in postmenopausal women at high fracture risk found them to be therapeutically equivalent. Both provided significant improvements in lumber spine BMD, TBS, and other parameters of bone health, assessed using multiple diagnostic methods.
Purpose: To compare the therapeutic efficacy of a teriparatide biosimilar (RGB-10) with reference teriparatide for the treatment of osteoporosis in postmenopausal women at very high fracture risk.
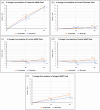
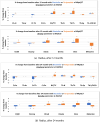
Methods: A retrospective analysis of 25 postmenopausal female patients treated for osteoporosis with RGB-10 for 24 months and a matched cohort of 25 patients treated with reference teriparatide. The following outcomes were assessed at baseline, 12 and 24 months: bone mineral density (BMD) at the lumbar spine, femoral neck and total hip using dual-energy x-ray absorptiometry (DXA) and integral, trabecular and cortical volumetric and surface BMD using 3D-SHAPER® imaging, trabecular bone score (TBS), quantitative ultrasound (QUS) measurements, and high-resolution peripheral quantitative computed tomography (HRpQCT) imaging of the radius and tibia.
Results: No significant differences were observed between treatment groups in any of the measured parameters of BMD or bone health at baseline as well as in any timepoint when assessed using these various diagnostic methods. Both compounds provided equivalent significant improvements from baseline in measures of osteoporosis and fracture risk.
Conclusion: The results of the analysis demonstrate the therapeutic equivalence of the teriparatide biosimilar (RGB-10) to reference teriparatide for the treatment of osteoporosis in postmenopausal women at very high risk of fracture.
Keywords: BMD; Biosimilar; Fracture; HRpQCT; Osteoporosis; Teriparatide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in accordance with the Declaration of Helsinki as well as with local ethical requirements. All study subjects gave written consent to participate. Conflicts of interest: P. Hadji: Has received study, educational and travel support from Amgen, Elli Lilly, Gedeon Richter, STADA Arzneimittel AG, Theramex and UCB. L. Kamali: No conflicts of interest relevant to this work. F. Thomasius: Has received honoraria for consulting or lectures from Amgen, Fresenius, Gedeon-Richter, STADA Arzneimittel AG, Theramex and UCB. K. Horas: No conflicts of interest relevant to this work. A. Kurth: Has received fees as a scientific advisor for Amgen, Theramex, Merit Medical, AgNovos, UCB, Image Biopsie Lab and as a lecturer for Amgen, STADA Arzneimittel AG, UCB, Alexion, AgNovos, Hologic. N. Bock: No conflicts of interest relevant to this work.
Figures


References
-
- Handel MN, Cardoso I, von Bulow C et al (2023) Fracture risk reduction and safety by osteoporosis treatment compared with placebo or active comparator in postmenopausal women: systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials. BMJ 381:e068033 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

