Developmental pluripotency-associated 4 increases aggressiveness of pituitary neuroendocrine tumors by enhancing cell stemness
- PMID: 39093695
- PMCID: PMC11726338
- DOI: 10.1093/neuonc/noae148
Developmental pluripotency-associated 4 increases aggressiveness of pituitary neuroendocrine tumors by enhancing cell stemness
Abstract
Background: Pituitary neuroendocrine tumors, PitNETs, are often aggressive and precipitate in distant metastases that are refractory to current therapies. However, the molecular mechanism in PitNETs' aggressiveness is not well understood. Developmental pluripotency-associated 4 (DPPA4) is known as a stem cell regulatory gene and overexpressed in certain cancers, but its function in the context of PitNETs' aggressiveness is not known.
Methods: We employed both rat and human models of PitNETs. In the rat pituitary tumor model, we used prenatal-alcohol-exposed (PAE) female Fischer rats which developed aggressive PitNETs following estrogen treatment, while in the human pituitary tumor model, we used aggressively proliferative cells from pituitary tumors of patients undergone surgery. Various molecular, cellular, and epigenetic techniques were used to determine the role of DPPA4 in PitNETs' aggressiveness.
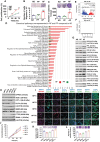
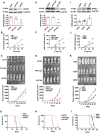
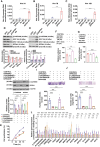
Results: We show that DPPA4 is overexpressed in association with increased cell stemness factors in aggressive PitNETs of PAE rats and of human patients. Gene-editing experiments demonstrate that DPPA4 increases the expression of cell stemness and tumor aggressiveness genes and promotes proliferation, colonization, migration, and tumorigenic potential of PitNET cells. ChIP assays and receptor antagonism studies reveal that DPPA4 binds to canonical WINTs promoters and increases directly or indirectly the WNT/β-CATENIN control of cell stemness, tumor growth, and aggressiveness of PitNETs. Epigenetic studies show the involvement of histone methyltransferase in alcohol activation of DPPA4.
Conclusions: These findings support a role of DPPA4 in tumor stemness and aggressiveness and provide a preclinical rationale for modulating this stemness regulator for the treatment of PitNETs.
Keywords: DPPA4; WNT/β-CATENIN; histone methyltransferase; pituitary neuroendocrine tumors; prenatal alcohol.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Colao A. Pituitary tumours: the prolactinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(5):575–596. - PubMed
-
- Raverot G, Burman P, McCormack A, et al.; European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1–G24. - PubMed
-
- Neou M, Villa C, Armignacco R, et al.Pangenomic classification of pituitary Neuroendocrine tumors. Cancer Cell. 2020;37(1):123–134.e5. - PubMed
-
- Lasolle H, Ilie MD, Raverot G.. Aggressive prolactinomas: how to manage? Pituitary. 2020;23(1):70–77. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

