Beneficial and harmful effects of tricyclic antidepressants for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis
- PMID: 39093721
- PMCID: PMC10806869
- DOI: 10.1136/bmjment-2023-300730
Beneficial and harmful effects of tricyclic antidepressants for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis
Abstract
Question: Tricyclic antidepressants are used to treat depression worldwide, but the adverse effects have not been systematically assessed. Our objective was to assess the beneficial and harmful effects of all tricyclic antidepressants for adults with major depressive disorder.
Study selection and analysis: We conducted a systematic review with meta-analysis and trial sequential analysis. We searched CENTRAL, MEDLINE, Embase, LILACS and other sources from inception to January 2023 for randomised clinical trials comparing tricyclic antidepressants versus placebo or 'active placebo' for adults with major depressive disorder. The primary outcomes were depressive symptoms measured on the 17-item Hamilton Depression Rating Scale (HDRS-17), serious adverse events and quality of life. The minimal important difference was defined as three points on the HDRS-17.
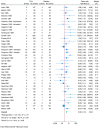
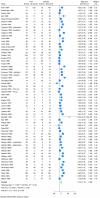
Findings: We included 103 trials randomising 10 590 participants. All results were at high risk of bias, and the certainty of the evidence was very low or low. All trials only assessed outcomes at the end of the treatment period at a maximum of 12 weeks after randomisation. Meta-analysis and trial sequential analysis showed evidence of a beneficial effect of tricyclic antidepressants compared with placebo (mean difference -3.77 HDRS-17 points; 95% CI -5.91 to -1.63; 17 trials). Meta-analysis showed evidence of a harmful effect of tricyclic antidepressants compared with placebo on serious adverse events (OR 2.78; 95% CI 2.18 to 3.55; 35 trials), but the required information size was not reached. Only 2 out of 103 trials reported on quality of life and t-tests showed no evidence of a difference.
Conclusions: The long-term effects of tricyclic antidepressants and the effects on quality of life are unknown. Short-term results suggest that tricyclic antidepressants may reduce depressive symptoms while also increasing the risks of serious adverse events, but these results were based on low and very low certainty evidence.
Prospero registration number: CRD42021226161.
Keywords: adult psychiatry; depression & mood disorders; psychiatry.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; MAH is a co-applicant and member of the DSMB of the RELEASE trial in Australia, funded by the Medical Research Future Fund (MRFF). MAH is co-founder and consultant for Outro Health, a digital clinic helping patients to stop unnecessary antidepressant medication. MAH has been paid honoraria by several NHS Trusts for grand rounds presentations, and by Salomon’s University and the University of Washington. MAH is a member of the Critical Psychiatry Network and the International Institute of Psychiatric Drug Withdrawal (IIPDW). JM is a co-investigator on REDUCE (programme grant studying discontinuation of antidepressants) and Chief Investigator on RADAR (programme grant to explore antipsychotic reduction and discontinuation). JM has been paid honoraria by University of Basel, Alberta Psychiatric Association, and Case Western University. JM receives royalties from Palgrave Macmillan and PCCS Books for three books about psychiatric drugs. JM is a co-chair person (unfunded position) of Critical Psychiatry Network. MPH receives royalties from Palgrave Macmillan for a book about antidepressants; no other relationships or activities that could appear to have influenced the submitted work.
Figures



Similar articles
-
The risks of adverse events with mirtazapine for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis.BMC Psychiatry. 2025 Jan 22;25(1):67. doi: 10.1186/s12888-024-06396-6. BMC Psychiatry. 2025. PMID: 39844067 Free PMC article.
-
Tricyclic antidepressants versus 'active placebo', placebo or no intervention for adults with major depressive disorder: a protocol for a systematic review with meta-analysis and Trial Sequential Analysis.Syst Rev. 2021 Aug 13;10(1):227. doi: 10.1186/s13643-021-01789-0. Syst Rev. 2021. PMID: 34389045 Free PMC article.
-
Beneficial and harmful effects of duloxetine versus placebo, 'active placebo' or no intervention for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis of randomised clinical trials.BMJ Open. 2025 Feb 7;15(2):e082853. doi: 10.1136/bmjopen-2023-082853. BMJ Open. 2025. PMID: 39920066 Free PMC article.
-
Beneficial and harmful effects of antidepressants versus placebo, 'active placebo', or no intervention for adults with major depressive disorder: a protocol for a systematic review of published and unpublished data with meta-analyses and trial sequential analyses.Syst Rev. 2021 May 25;10(1):154. doi: 10.1186/s13643-021-01705-6. Syst Rev. 2021. PMID: 34034811 Free PMC article.
-
Antidepressants for the treatment of depression in people with cancer.Cochrane Database Syst Rev. 2023 Mar 31;3(3):CD011006. doi: 10.1002/14651858.CD011006.pub4. Cochrane Database Syst Rev. 2023. PMID: 36999619 Free PMC article. Review.
Cited by
-
The risks of adverse events with venlafaxine for adults with major depressive disorder: a systematic review of randomised clinical trials with meta-analysis and Trial Sequential Analysis.Epidemiol Psychiatr Sci. 2024 Oct 23;33:e51. doi: 10.1017/S2045796024000520. Epidemiol Psychiatr Sci. 2024. PMID: 39440379 Free PMC article.
-
Lewy body dementia: exploring biomarkers and pathogenic interactions of amyloid β, tau, and α-synuclein.Mol Neurodegener. 2025 Aug 12;20(1):90. doi: 10.1186/s13024-025-00879-0. Mol Neurodegener. 2025. PMID: 40797227 Free PMC article. Review.
-
Chemotherapy-Induced Peripheral Neuropathy: A Recent Update on Pathophysiology and Treatment.Life (Basel). 2024 Aug 9;14(8):991. doi: 10.3390/life14080991. Life (Basel). 2024. PMID: 39202733 Free PMC article. Review.
-
The risks of adverse events with mirtazapine for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis.BMC Psychiatry. 2025 Jan 22;25(1):67. doi: 10.1186/s12888-024-06396-6. BMC Psychiatry. 2025. PMID: 39844067 Free PMC article.
-
Missing outcome data in randomised clinical trials of psychological interventions: a review of published trial reports in major psychiatry journals.BMC Psychiatry. 2024 Nov 14;24(1):798. doi: 10.1186/s12888-024-06263-4. BMC Psychiatry. 2024. PMID: 39543512 Free PMC article. Review.
References
-
- American Psychiatric Association . Diagnostic and statistical Manual of mental disorders. In: Diagnostic and statistical manual of mental disorders (DSM-5®). Washington DC: American Psychiatric Publishing, 2013. 10.1176/appi.books.9780890425596 - DOI
-
- Swann T. “'anarchist Technologies': anarchism, cybernetics and mutual aid in community responses to the COVID-19 crisis”. Organization (Lond) 2023;30:193–209. 10.1177/13505084221090632 Available: https://www.who.int/news-room/fact-sheets/detail/depression - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
