Caspase-2 is essential for proliferation and self-renewal of nucleophosmin-mutated acute myeloid leukemia
- PMID: 39093977
- PMCID: PMC11296348
- DOI: 10.1126/sciadv.adj3145
Caspase-2 is essential for proliferation and self-renewal of nucleophosmin-mutated acute myeloid leukemia
Erratum in
-
Erratum for the Research Article: "Caspase-2 is essential for proliferation and self-renewal of nucleophosmin-mutated acute myeloid leukemia" by D. Sakthivel et al.Sci Adv. 2024 Oct 18;10(42):eadt3858. doi: 10.1126/sciadv.adt3858. Epub 2024 Oct 18. Sci Adv. 2024. PMID: 39423280 Free PMC article. No abstract available.
Abstract
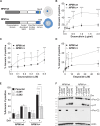
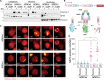
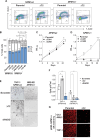
Mutation in nucleophosmin (NPM1) causes relocalization of this normally nucleolar protein to the cytoplasm (NPM1c+). Despite NPM1 mutation being the most common driver mutation in cytogenetically normal adult acute myeloid leukemia (AML), the mechanisms of NPM1c+-induced leukemogenesis remain unclear. Caspase-2 is a proapoptotic protein activated by NPM1 in the nucleolus. Here, we show that caspase-2 is also activated by NPM1c+ in the cytoplasm and DNA damage-induced apoptosis is caspase-2 dependent in NPM1c+ but not in NPM1wt AML cells. Strikingly, in NPM1c+ cells, caspase-2 loss results in profound cell cycle arrest, differentiation, and down-regulation of stem cell pathways that regulate pluripotency including impairment of the AKT/mTORC1 pathways, and inhibition of Rictor cleavage. In contrast, there were minimal differences in proliferation, differentiation, or the transcriptional profile of NPM1wt cells lacking caspase-2. Our results show that caspase-2 is essential for proliferation and self-renewal of AML cells expressing mutated NPM1. This study demonstrates that caspase-2 is a major effector of NPM1c+ function.
Figures



Update of
-
Caspase-2 is essential for proliferation and self-renewal of nucleophosmin-mutated acute myeloid leukemia.bioRxiv [Preprint]. 2023 May 30:2023.05.29.542723. doi: 10.1101/2023.05.29.542723. bioRxiv. 2023. Update in: Sci Adv. 2024 Aug 2;10(31):eadj3145. doi: 10.1126/sciadv.adj3145. PMID: 37398413 Free PMC article. Updated. Preprint.
References
-
- A. M. Noone, N. Howlader, M. Krapcho, D. Miller, A. Brest, M. Yu, J. Ruhl, Z. Tatalovich, A. Mariotto, D. R. Lewis, H. S. Chen, E. J. Feuer, K. A. Cronin, Eds. SEER Cancer Statistics Review, 1975-2015 (National Cancer Institute, 2018); https://seer.cancer.gov/csr/1975_2015/.
-
- Falini B., Mecucci C., Tiacci E., Alcalay M., Rosati R., Pasqualucci L., la Starza R., Diverio D., Colombo E., Santucci A., Bigerna B., Pacini R., Pucciarini A., Liso A., Vignetti M., Fazi P., Meani N., Pettirossi V., Saglio G., Mandelli F., Lo-Coco F., Pelicci P. G., Martelli M. F., Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 352, 254–266 (2005). - PubMed
-
- Grisendi S., Mecucci C., Falini B., Pandolfi P. P., Nucleophosmin and cancer. Nat. Rev. Cancer 6, 493–505 (2006). - PubMed
-
- Arber D. A., Orazi A., Hasserjian R. P., Borowitz M. J., Calvo K. R., Kvasnicka H. M., Wang S. A., Bagg A., Barbui T., Branford S., Bueso-Ramos C. E., Cortes J. E., Dal Cin P., DiNardo C. D., Dombret H., Duncavage E. J., Ebert B. L., Estey E. H., Facchetti F., Foucar K., Gangat N., Gianelli U., Godley L. A., Gökbuget N., Gotlib J., Hellström-Lindberg E., Hobbs G. S., Hoffman R., Jabbour E. J., Kiladjian J. J., Larson R. A., le Beau M. M., Loh M. L. C., Löwenberg B., Macintyre E., Malcovati L., Mullighan C. G., Niemeyer C., Odenike O. M., Ogawa S., Orfao A., Papaemmanuil E., Passamonti F., Porkka K., Pui C. H., Radich J. P., Reiter A., Rozman M., Rudelius M., Savona M. R., Schiffer C. A., Schmitt-Graeff A., Shimamura A., Sierra J., Stock W. A., Stone R. M., Tallman M. S., Thiele J., Tien H. F., Tzankov A., Vannucchi A. M., Vyas P., Wei A. H., Weinberg O. K., Wierzbowska A., Cazzola M., Döhner H., Tefferi A., International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 140, 1200–1228 (2022). - PMC - PubMed
-
- Martelli M. P., Gionfriddo I., Mezzasoma F., Milano F., Pierangeli S., Mulas F., Pacini R., Tabarrini A., Pettirossi V., Rossi R., Vetro C., Brunetti L., Sportoletti P., Tiacci E., di Raimondo F., Falini B., Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 125, 3455–3465 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

