MiR-34b promotes oxidative stress and induces cellular senescence through TWIST1 in human cervical cancer
- PMID: 39094513
- PMCID: PMC11342277
- DOI: 10.1016/j.tranon.2024.102063
MiR-34b promotes oxidative stress and induces cellular senescence through TWIST1 in human cervical cancer
Abstract
Purpose: The aim of this research was to elucidate the role of miR-34b in cervical cancer progression and the underlying mechanism behind the miR-34b-mediated tumor suppression. The study revealed the role of miR-34b as a senescence inducer and serves as a potential therapeutic target in developing combination therapy with senotherapeutics.
Methods: MiR-34b was ectopically expressed in cervical cancer cell lines using a tetracycline inducible system and its effects on cell viability, apoptosis, senescence, DNA damage and oxidative stress were studied using MTT assay, acridine orange/ ethidium bromide staining, senescence associated β-galactosidase assay, gamma H2AX foci staining assay, western blotting and specific dyes for the detection of total and individual ROS species.
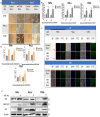
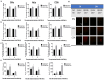
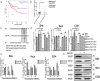
Results: Ectopic expression of miR-34b promoted cellular senescence but no significant induction of apoptosis was observed in cervical cancer cell lines. MiR-34b promoted increase in oxidative stress through increase in total and individual ROS species and contributed to increase in cellular senescence. Mechanistically, miR-34b mediates its action by targeting TWIST1 as evidenced by the similar actions of TWIST1 shRNA in cervical cancer cell lines. Furthermore, our study revealed TWIST1 is one of the most significant targets of miR-34b targetome and identified RITA as a novel senolytic agent for use in combination therapy with miR-34b.
Conclusion: MiR-34b promotes cellular senescence and oxidative stress by targeting TWIST1, a known oncogene and EMT regulator. This study delved into the mechanism of miR-34b-mediated tumor suppression and provided novel insights for development of miR-34b based therapeutics for cervical cancer.
Keywords: Cervical cancer; MiR-34b; Oxidative stress; Senescence; TWIST1.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




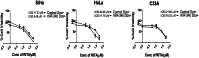
Similar articles
-
MiR-34b regulates cervical cancer cell proliferation and apoptosis.Artif Cells Nanomed Biotechnol. 2019 Dec;47(1):2042-2047. doi: 10.1080/21691401.2019.1614013. Artif Cells Nanomed Biotechnol. 2019. PMID: 31119955
-
IDH2 Deficiency Promotes Endothelial Senescence by Eliciting miR-34b/c-Mediated Suppression of Mitophagy and Increased ROS Production.Antioxidants (Basel). 2023 Feb 27;12(3):585. doi: 10.3390/antiox12030585. Antioxidants (Basel). 2023. PMID: 36978833 Free PMC article.
-
miR-34b inhibits nasopharyngeal carcinoma cell proliferation by targeting ubiquitin-specific peptidase 22.Onco Targets Ther. 2016 Mar 16;9:1525-34. doi: 10.2147/OTT.S98378. eCollection 2016. Onco Targets Ther. 2016. PMID: 27051294 Free PMC article.
-
miR-34b-3p-mediated regulation of STC2 and FN1 enhances chemosensitivity and inhibits proliferation in cervical cancer.Acta Biochim Biophys Sin (Shanghai). 2024 May 25;56(5):740-752. doi: 10.3724/abbs.2024009. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38477044 Free PMC article.
-
Role of microRNA-34b-5p in cancer and injury: how does it work?Cancer Cell Int. 2022 Dec 1;22(1):381. doi: 10.1186/s12935-022-02797-3. Cancer Cell Int. 2022. PMID: 36457043 Free PMC article. Review.
Cited by
-
Cellular senescence in cancer: from mechanism paradoxes to precision therapeutics.Mol Cancer. 2025 Aug 8;24(1):213. doi: 10.1186/s12943-025-02419-2. Mol Cancer. 2025. PMID: 40781676 Free PMC article. Review.
References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R.L., Soerjomataram I., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [Internet] Available from. - DOI - PubMed
-
- Balasubramaniam G., Gaidhani R.H., Khan A., Saoba S., Mahantshetty U., Maheshwari A. Survival rate of cervical cancer from a study conducted in India. Indian J. Med. Sci. 2020;73:203–211. doi: 10.1016/j.lansea.2023.100296. [Internet] Available from. - DOI
LinkOut - more resources
Full Text Sources

