Induction of astrocyte reactivity promotes neurodegeneration in human pluripotent stem cell models
- PMID: 39094561
- PMCID: PMC11368677
- DOI: 10.1016/j.stemcr.2024.07.002
Induction of astrocyte reactivity promotes neurodegeneration in human pluripotent stem cell models
Abstract
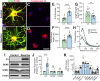
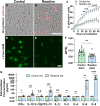
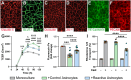
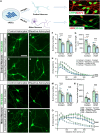
Reactive astrocytes are known to exert detrimental effects upon neurons in several neurodegenerative diseases, yet our understanding of how astrocytes promote neurotoxicity remains incomplete, especially in human systems. In this study, we leveraged human pluripotent stem cell (hPSC) models to examine how reactivity alters astrocyte function and mediates neurodegeneration. hPSC-derived astrocytes were induced to a reactive phenotype, at which point they exhibited a hypertrophic profile and increased complement C3 expression. Functionally, reactive astrocytes displayed decreased intracellular calcium, elevated phagocytic capacity, and decreased contribution to the blood-brain barrier. Subsequently, co-culture of reactive astrocytes with a variety of neuronal cell types promoted morphological and functional alterations. Furthermore, when reactivity was induced in astrocytes from patient-specific hPSCs (glaucoma, Alzheimer's disease, and amyotrophic lateral sclerosis), the reactive state exacerbated astrocytic disease-associated phenotypes. These results demonstrate how reactive astrocytes modulate neurodegeneration, significantly contributing to our understanding of a role for reactive astrocytes in neurodegenerative diseases.
Keywords: astrocyte; differentiation; disease; neurodegeneration; reactivity; stem cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.S.M. holds a patent related to methods for the retinal differentiation of human pluripotent stem cells used in this study.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

