A replisome-associated histone H3-H4 chaperone required for epigenetic inheritance
- PMID: 39094570
- PMCID: PMC11380579
- DOI: 10.1016/j.cell.2024.07.006
A replisome-associated histone H3-H4 chaperone required for epigenetic inheritance
Abstract
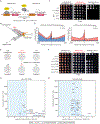
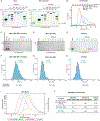
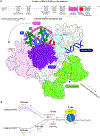
Faithful transfer of parental histones to newly replicated daughter DNA strands is critical for inheritance of epigenetic states. Although replication proteins that facilitate parental histone transfer have been identified, how intact histone H3-H4 tetramers travel from the front to the back of the replication fork remains unknown. Here, we use AlphaFold-Multimer structural predictions combined with biochemical and genetic approaches to identify the Mrc1/CLASPIN subunit of the replisome as a histone chaperone. Mrc1 contains a conserved histone-binding domain that forms a brace around the H3-H4 tetramer mimicking nucleosomal DNA and H2A-H2B histones, is required for heterochromatin inheritance, and promotes parental histone recycling during replication. We further identify binding sites for the FACT histone chaperone in Swi1/TIMELESS and DNA polymerase α that are required for heterochromatin inheritance. We propose that Mrc1, in concert with FACT acting as a mobile co-chaperone, coordinates the distribution of parental histones to newly replicated DNA.
Keywords: CLASPIN; FACT; chromatin replication; epigenetics; fork protection complex; heterochromatin; histone inheritance; parental histone transfer; replisome, Mrc1.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

