Viral hijacking of hnRNPH1 unveils a G-quadruplex-driven mechanism of stress control
- PMID: 39094585
- PMCID: PMC12207933
- DOI: 10.1016/j.chom.2024.07.006
Viral hijacking of hnRNPH1 unveils a G-quadruplex-driven mechanism of stress control
Abstract
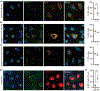
Viral genomes are enriched with G-quadruplexes (G4s), non-canonical structures formed in DNA or RNA upon assembly of four guanine stretches into stacked quartets. Because of their critical roles, G4s are potential antiviral targets, yet their function remains largely unknown. Here, we characterize the formation and functions of a conserved G4 within the polymerase coding region of orthoflaviviruses of the Flaviviridae family. Using yellow fever virus, we determine that this G4 promotes viral replication and suppresses host stress responses via interactions with hnRNPH1, a host nuclear protein involved in RNA processing. G4 binding to hnRNPH1 causes its cytoplasmic retention with subsequent impacts on G4-containing tRNA fragments (tiRNAs) involved in stress-mediated reductions in translation. As a result, these host stress responses and associated antiviral effects are impaired. These data reveal that the interplay between hnRNPH1 and both host and viral G4 targets controls the integrated stress response and viral replication.
Keywords: G-quadruplex; antiviral stress response; hnRNPH1; host factor; orthoflavivirus; tiRNA.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
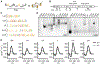
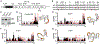




References
-
- Postler TS, Beer M, Blitvich BJ, Bukh J, de Lamballerie X, Drexler JF, Imrie A, Kapoor A, Karganova GG, Lemey P, et al. (2023). Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch. Virol 168, 224. 10.1007/s00705-023-05835-1. - DOI - PubMed
-
- Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, Perea W, and Ferguson NM; Yellow; Fever; Expert Committee (2014). Yellow fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 11, e1001638. 10.1371/journal.pmed.1001638. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

