SCD4 deficiency decreases cardiac steatosis and prevents cardiac remodeling in mice fed a high-fat diet
- PMID: 39094772
- PMCID: PMC11402454
- DOI: 10.1016/j.jlr.2024.100612
SCD4 deficiency decreases cardiac steatosis and prevents cardiac remodeling in mice fed a high-fat diet
Abstract
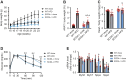
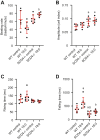
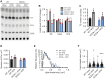
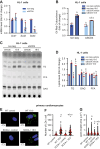
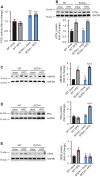
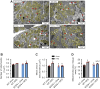
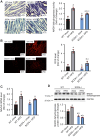
Stearoyl-CoA desaturase (SCD) is a lipogenic enzyme that catalyzes formation of the first double bond in the carbon chain of saturated fatty acids. Four isoforms of SCD have been identified in mice, the most poorly characterized of which is SCD4, which is cardiac-specific. In the present study, we investigated the role of SCD4 in systemic and cardiac metabolism. We used WT and global SCD4 KO mice that were fed standard laboratory chow or a high-fat diet (HFD). SCD4 deficiency reduced body adiposity and decreased hyperinsulinemia and hypercholesterolemia in HFD-fed mice. The loss of SCD4 preserved heart morphology in the HFD condition. Lipid accumulation decreased in the myocardium in SCD4-deficient mice and in HL-1 cardiomyocytes with knocked out Scd4 expression. This was associated with an increase in the rate of lipolysis and, more specifically, adipose triglyceride lipase (ATGL) activity. Possible mechanisms of ATGL activation by SCD4 deficiency include lower protein levels of the ATGL inhibitor G0/G1 switch protein 2 and greater activation by protein kinase A under lipid overload conditions. Moreover, we observed higher intracellular Ca2+ levels in HL-1 cells with silenced Scd4 expression. This may explain the activation of protein kinase A in response to higher Ca2+ levels. Additionally, the loss of SCD4 inhibited mitochondrial enlargement, NADH overactivation, and reactive oxygen species overproduction in the heart in HFD-fed mice. In conclusion, SCD4 deficiency activated lipolysis, resulting in a reduction of cardiac steatosis, prevented the induction of left ventricular hypertrophy, and reduced reactive oxygen species levels in the heart in HFD-fed mice.
Keywords: ATGL; heart; lipid droplets; metabolism; mitochondria.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Belke D.D., Larsen T.S., Lopaschuk G.D., Severson D.L. Glucose and fatty acid metabolism in the isolated working mouse heart. Am. J. Physiol. 1999;277:R1210–R1217. - PubMed
-
- Ntambi J.M., Buhrow S.A., Kaestner K.H., Christy R.J., Sibley E., Kelly T.J., et al. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 1988;263:17291–17300. - PubMed
-
- Kaestner K.H., Ntambi J.M., Kelly T.J., Lane M.D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. A second differentially expressed gene encoding stearoyl-CoA desaturase. J. Biol. Chem. 1989;264:14755–14761. - PubMed
-
- Zheng Y., Prouty S.M., Harmon A., Sundberg J.P., Stenn K.S., Parimoo S. Scd3 - a novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics. 2001;71:182–191. - PubMed
-
- Miyazaki M., Jacobson M.J., Man W.C., Cohen P., Asilmaz E., Friedman J.M., et al. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 2003;278:33904–33911. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

