The molecular mechanism of NF-κB dysregulation across different subtypes of renal cell carcinoma
- PMID: 39094893
- PMCID: PMC12147641
- DOI: 10.1016/j.jare.2024.07.030
The molecular mechanism of NF-κB dysregulation across different subtypes of renal cell carcinoma
Abstract
Background: The nuclear factor kappa B (NF-κB) is a critical pathway that regulates various cellular functions, including immune response, proliferation, growth, and apoptosis. Furthermore, this pathway is tightly regulated to ensure stability in the presence of immunogenic triggers or genotoxic stimuli. The lack of control of the NF-κB pathway can lead to the initiation of different diseases, mainly autoimmune diseases and cancer, including Renal cell carcinoma (RCC). RCC is the most common type of kidney cancer and is characterized by complex genetic composition and elusive molecular mechanisms.
Aim of review: The current review summarizes the mechanism of NF-κB dysregulation in different subtypes of RCC and its impact on pathogenesis.
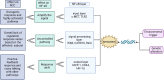
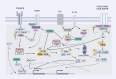
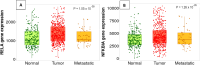
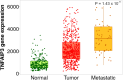
Key scientific concept of review: This review highlights the prominent role of NF-κB in RCC development and progression by driving the expression of multiple genes and interplaying with different pathways, including the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway. In silico analysis of RCC cohorts and molecular studies have revealed that multiple NF-κB members and target genes are dysregulated. The dysregulation includes receptors such as TLR2, signal-transmitting members including RelA, and target genes, for instance, HIF-1α. The lack of effective regulatory mechanisms results in a constitutively active NF-κB pathway, which promotes cancer growth, migration, and survival. In this review, we comprehensively summarize the role of dysregulated NF-κB-related genes in the most common subtypes of RCC, including clear cell RCC (ccRCC), chromophobe RCC (chRCC), and papillary RCC (PRCC).
Keywords: Genetic modification; Molecular mechanism; NF-κB dysregulation; Renal cell carcinoma.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The interaction of YBX1 with G3BP1 promotes renal cell carcinoma cell metastasis via YBX1/G3BP1-SPP1- NF-κB signaling axis.J Exp Clin Cancer Res. 2019 Sep 3;38(1):386. doi: 10.1186/s13046-019-1347-0. J Exp Clin Cancer Res. 2019. PMID: 31481087 Free PMC article.
-
The emerging role of nuclear factor kappa B in renal cell carcinoma.Int J Biochem Cell Biol. 2011 Nov;43(11):1537-49. doi: 10.1016/j.biocel.2011.08.003. Epub 2011 Aug 12. Int J Biochem Cell Biol. 2011. PMID: 21854869 Review.
-
Nuclear factor-kappa B subunits and their prognostic cancer-specific survival value in renal cell carcinoma patients.Pathology. 2018 Aug;50(5):511-518. doi: 10.1016/j.pathol.2018.03.003. Epub 2018 Jun 21. Pathology. 2018. PMID: 29935727
-
Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT → NF-κB → CXCL5 signaling.Sci Rep. 2016 Nov 16;6:37085. doi: 10.1038/srep37085. Sci Rep. 2016. PMID: 27848972 Free PMC article.
-
The Role of the PAX Genes in Renal Cell Carcinoma.Int J Mol Sci. 2024 Jun 19;25(12):6730. doi: 10.3390/ijms25126730. Int J Mol Sci. 2024. PMID: 38928435 Free PMC article. Review.
Cited by
-
Serum Starvation Enhances the Antitumor Activity of Natural Matrices: Insights into Bioactive Molecules from Dromedary Urine Extracts.Molecules. 2025 Feb 10;30(4):821. doi: 10.3390/molecules30040821. Molecules. 2025. PMID: 40005133 Free PMC article.
-
Integrative Transcriptome-Wide Association Study With Expression Quantitative Trait Loci Colocalization Identifies a Causal VAMP8 Variant for Nasopharyngeal Carcinoma Susceptibility.Adv Sci (Weinh). 2025 Mar;12(11):e2412580. doi: 10.1002/advs.202412580. Epub 2025 Jan 24. Adv Sci (Weinh). 2025. PMID: 39854120 Free PMC article.
-
Exosomes derived from FN14-overexpressing BMSCs activate the NF-κB signaling pathway to induce PANoptosis in osteosarcoma.Apoptosis. 2025 Apr;30(3-4):880-893. doi: 10.1007/s10495-024-02071-z. Epub 2025 Jan 20. Apoptosis. 2025. PMID: 39833632 Free PMC article.
-
Molecular Mechanisms and Therapeutic Role of Intra-Articular Hyaluronic Acid in Osteoarthritis: A Precision Medicine Perspective.J Clin Med. 2025 Apr 8;14(8):2547. doi: 10.3390/jcm14082547. J Clin Med. 2025. PMID: 40283379 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

