Primaquine-5,6-Orthoquinone Is Directly Hemolytic to Older G6PD Deficient RBCs in a Humanized Mouse Model
- PMID: 39095205
- PMCID: PMC11413921
- DOI: 10.1124/jpet.124.002218
Primaquine-5,6-Orthoquinone Is Directly Hemolytic to Older G6PD Deficient RBCs in a Humanized Mouse Model
Abstract
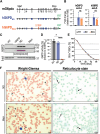
Primaquine and Tafenoquine are the only approved drugs that can achieve a radical cure for Plasmodium vivax malaria but are contraindicated in patients who are deficient in glucose 6-phosphate dehydrogenase (G6PDd) due to risk of severe hemolysis from reactive oxygen species generated by redox cycling of drug metabolites. 5-hydroxyprimaquine and its quinoneimine cause robust redox cycling in red blood cells (RBCs) but are so labile as to not be detected in blood or urine. Rather, the quinoneimine is rapidly converted into primaquine-5,6-orthoquinone (5,6-POQ) that is then excreted in the urine. The extent to which 5,6-POQ contributes to hemolysis remains unclear, although some have suggested that it is a minor toxin that should be used predominantly as a surrogate to infer levels of 5-hydroxyprimaquine. In this report, we describe a novel humanized mouse model of the G6PD Mediterranean variant (hG6PDMed-) that recapitulates the human biology of RBC age-dependent enzyme decay, as well as an isogenic matched control mouse with human nondeficient G6PD hG6PDND In vitro challenge of RBCs with 5,6-POQ causes increased generation of superoxide and methemoglobin. Infusion of treated RBCs shows that 5,6-POQ selectively causes in vivo clearance of older hG6PDMed- RBCs. These findings support the hypothesis that 5,6-POQ directly induces hemolysis and challenges the notion that 5,6-POQ is an inactive metabolic waste product. Indeed, given the extreme lability of 5-hydroxyprimaquine and the relative stability of 5,6-POQ, these data raise the possibility that 5,6-POQ is a major hemolytic primaquine metabolite in vivo. SIGNIFICANCE STATEMENT: These findings demonstrate that 5,6-POQ, which has been considered an inert waste product of primaquine metabolism, directly induces ROS that cause clearance of older G6PDd RBCs. As 5,6-POQ is relatively stable compared with other active primaquine metabolites, these data support the hypothesis that 5,6-POQ is a major toxin in primaquine induced hemolysis. The findings herein also establish a new model of G6PDd and provide the first direct evidence, to our knowledge, that young G6PDd RBCs are resistant to primaquine-induced hemolysis.
Copyright © 2024 by The American Society for Pharmacology and Experimental Therapeutics.
Figures





References
-
- Agarwal S, Gupta UR, Daniel CS, Gupta RC, Anand N, Agarwal SS (1991) Susceptibility of glucose-6-phosphate dehydrogenase deficient red cells to primaquine, primaquine enantiomers, and its two putative metabolites. II. Effect on red blood cell membrane, lipid peroxidation, MC-540 staining, and scanning electron microscopic studies. Biochem Pharmacol 41:17–21. - PubMed
-
- Agarwal S, Gupta UR, Gupta RC, Anand N, Agarwal SS (1988) Susceptibility of glucose-6-phosphate dehydrogenase deficient red cells to primaquine enantiomers and two putative metabolites–I. Effect on reduced glutathione, methemoglobin content and release of hemoglobin. Biochem Pharmacol 37:4605–4609. - PubMed
-
- Au SW, Gover S, Lam VM, Adams MJ (2000) Human glucose-6-phosphate dehydrogenase: the crystal structure reveals a structural NADP(+) molecule and provides insights into enzyme deficiency. Structure 8:293–303. - PubMed
-
- Augusto O, Schreiber J, Mason RP (1988) Direct ESR detection of a free radical intermediate during the peroxidase-catalyzed oxidation of the antimalarial drug primaquine. Biochem Pharmacol 37:2791–2797. - PubMed
-
- Avula BTekwani BLChaurasiya NDFasinu PDhammika Nanayakkara NPBhandara Herath HMTWang Y-HBae J-YKhan SIElsohly MA, et al. (2018) Metabolism of primaquine in normal human volunteers: investigation of phase I and phase II metabolites from plasma and urine using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Malar J 17:294. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
