Huntington's disease cellular phenotypes are rescued non-cell autonomously by healthy cells in mosaic telencephalic organoids
- PMID: 39095390
- PMCID: PMC11297310
- DOI: 10.1038/s41467-024-50877-x
Huntington's disease cellular phenotypes are rescued non-cell autonomously by healthy cells in mosaic telencephalic organoids
Erratum in
-
Author Correction: Huntington's disease cellular phenotypes are rescued non-cell autonomously by healthy cells in mosaic telencephalic organoids.Nat Commun. 2025 May 21;16(1):4723. doi: 10.1038/s41467-025-60108-6. Nat Commun. 2025. PMID: 40399310 Free PMC article. No abstract available.
Abstract
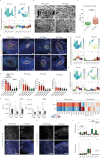
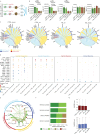
Huntington's disease (HD) causes selective degeneration of striatal and cortical neurons, resulting in cell mosaicism of coexisting still functional and dysfunctional cells. The impact of non-cell autonomous mechanisms between these cellular states is poorly understood. Here we generated telencephalic organoids with healthy or HD cells, grown separately or as mosaics of the two genotypes. Single-cell RNA sequencing revealed neurodevelopmental abnormalities in the ventral fate acquisition of HD organoids, confirmed by cytoarchitectural and transcriptional defects leading to fewer GABAergic neurons, while dorsal populations showed milder phenotypes mainly in maturation trajectory. Healthy cells in mosaic organoids restored HD cell identity, trajectories, synaptic density, and communication pathways upon cell-cell contact, while showing no significant alterations when grown with HD cells. These findings highlight cell-type-specific alterations in HD and beneficial non-cell autonomous effects of healthy cells, emphasizing the therapeutic potential of modulating cell-cell communication in disease progression and treatment.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures