CREB-regulated transcription during glycogen synthesis in astrocytes
- PMID: 39095513
- PMCID: PMC11297295
- DOI: 10.1038/s41598-024-67976-w
CREB-regulated transcription during glycogen synthesis in astrocytes
Abstract
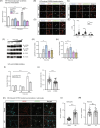
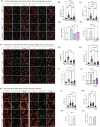
Glycogen storage, conversion and utilization in astrocytes play an important role in brain energy metabolism. The conversion of glycogen to lactate through glycolysis occurs through the coordinated activities of various enzymes and inhibition of this process can impair different brain processes including formation of long-lasting memories. To replenish depleted glycogen stores, astrocytes undergo glycogen synthesis, a cellular process that has been shown to require transcription and translation during specific stimulation paradigms. However, the detail nuclear signaling mechanisms and transcriptional regulation during glycogen synthesis in astrocytes remains to be explored. In this report, we study the molecular mechanisms of vasoactive intestinal peptide (VIP)-induced glycogen synthesis in astrocytes. VIP is a potent neuropeptide that triggers glycogenolysis followed by glycogen synthesis in astrocytes. We show evidence that VIP-induced glycogen synthesis requires CREB-mediated transcription that is calcium dependent and requires conventional Protein Kinase C but not Protein Kinase A. In parallel to CREB activation, we demonstrate that VIP also triggers nuclear accumulation of the CREB coactivator CRTC2 in astrocytic nuclei. Transcriptome profiles of VIP-induced astrocytes identified robust CREB transcription, including a subset of genes linked to glucose and glycogen metabolism. Finally, we demonstrate that VIP-induced glycogen synthesis shares similar as well as distinct molecular signatures with glucose-induced glycogen synthesis, including the requirement of CREB-mediated transcription. Overall, our data demonstrates the importance of CREB-mediated transcription in astrocytes during stimulus-driven glycogenesis.
Keywords: Astrocyte; CREB; Glycogen synthesis; Transcription; Vasoactive intestinal peptide.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- MOE2017-T3-1-002/Ministry of Education - Singapore
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
- Nanyang Assistant Professorship Program/Nanyang Technological University
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

