The impact of CLDN18.2 expression on effector cells mediating antibody-dependent cellular cytotoxicity in gastric cancer
- PMID: 39095563
- PMCID: PMC11297210
- DOI: 10.1038/s41598-024-68970-y
The impact of CLDN18.2 expression on effector cells mediating antibody-dependent cellular cytotoxicity in gastric cancer
Abstract
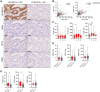
Activating antibody-dependent cellular cytotoxicity (ADCC) by targeting claudin-18 isoform 2 (CLDN18.2) using zolbetuximab, a monoclonal antibody against CLDN18.2, has been considered a promising novel therapeutic strategy for gastric cancer (GC). However, the impact of CLDN18.2 expression on natural killer (NK) cells and monocytes/macrophages-crucial effector cells of ADCC-in GC has not been fully investigated. In the present study, we assessed the impact of CLDN18.2 expression on clinical outcomes, molecular features, and the frequencies of tumor-infiltrating NK cells and macrophages, as well as peripheral blood NK cells and monocytes, in GC by analyzing our own GC cohorts. The expression of CLDN18.2 did not significantly impact clinical outcomes of GC patients, while it was significantly and positively associated with Epstein-Barr virus (EBV) status and PD-L1 expression. The frequencies of tumor-infiltrating NK cells and macrophages, as well as peripheral blood NK cells and monocytes, were comparable between CLDN18.2-positive and CLDN18.2-negative GCs. Importantly, both CLDN18.2 expression and the number of tumor-infiltrating NK cells were significantly higher in EBV-associated GC compared to other molecular subtypes. Our findings support the effectiveness of zolbetuximab in CLDN18.2-positive GC, and offer a novel insight into the treatment of this cancer type, highlighting its potential effectiveness for CLDN18.2-positive/EBV-associated GC.
Keywords: CLDN18.2; Gastric cancer; Monocytes/macrophages; NK cells.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Sahin, U. et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol.32, 609–619. 10.1016/j.annonc.2021.02.005 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

