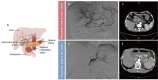
Digital subtraction angiography-guided pancreatic arterial infusion of GEMOX chemotherapy in advanced pancreatic adenocarcinoma: a phase II, open-label, randomized controlled trial comparing with intravenous chemotherapy
- PMID: 39095759
- PMCID: PMC11295591
- DOI: 10.1186/s12885-024-12695-8
Digital subtraction angiography-guided pancreatic arterial infusion of GEMOX chemotherapy in advanced pancreatic adenocarcinoma: a phase II, open-label, randomized controlled trial comparing with intravenous chemotherapy
Abstract
Background: Advanced pancreatic adenocarcinoma lacks effective treatment options, and systemic gemcitabine-based chemotherapy offers only marginal survival benefits at the cost of significant toxicities and adverse events. New therapeutic options with better drug availability are warranted. This study aims to evaluate the safety and efficacy of digital subtraction angiography (DSA)-guided pancreatic arterial infusion (PAI) versus intravenous chemotherapy (IVC) using the gemcitabine and oxaliplatin (GEMOX) regimen in unresectable locally advanced or metastatic pancreatic cancer (PC) patients.
Materials and methods: This study prospectively enrolled 51 eligible treatment-naive patients with unresectable PC to receive GEMOX treatment via PAI or IVC (1:1 ratio randomization) from December 2015 to December 2019. Cycles were repeated monthly, and each process consisted of two treatments administered bi-weekly. Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), 1-year survival, 6-month survival, tumor-site subgroup survival, and incidences of adverse events were compared.
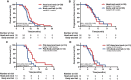
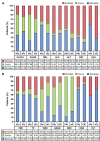
Results: The median OS of the PAI and IVC groups were 9.93 months and 10.07 months, respectively (p = 0.3049). The median PFS of the PAI and IVC groups were 5.07 months and 4.23 months (p = 0.1088). No significant differences were found in the ORR (11.54% vs. 4%, p = 0.6312), DCR (53.85% vs. 44%, p = 0.482), and 1-year OS rate (44% vs. 20.92%, p = 0.27) in PAI and IVC groups. The 6-month OS rate was significantly higher in the PAI group (100%) than in the IVC group (83.67%) (p = 0.0173). The median OS of patients in PAI group with pancreatic head and neck tumors were significantly higher than those of body and tail tumors (12.867 months vs. 9 months, p = 0.0214). The incidences of hematologic disorders, liver function disorders, and digestive disorders in the IVC group were higher than in the PAI group (p < 0.05).
Conclusion: GEMOX PAI therapy presented a higher 6-month OS rate and fewer adverse events than IVC in advanced pancreatic adenocarcinoma patients. Those with pancreatic head and neck tumors may yield a superior treatment outcome from PAI treatment.
Trial registration number: NCT02635971.
Date of registration: 21/12/2015.
Keywords: GEMOX; Intravenous chemotherapy; Pancreatic arterial infusion; Pancreatic head and neck tumors; Unresectable pancreatic cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Gadaleta CD, Solbiati L, Mattioli V, Rubini G, Fazio V, Goffredo V, Vinciarelli G, Gadaleta-Caldarola G, Canniello E, Armenise F, et al. Unresectable lung malignancy: combination therapy with segmental pulmonary arterial chemoembolization with drug-eluting microspheres and radiofrequency ablation in 17 patients. Radiology. 2013;267(2):627–37. 10.1148/radiol.12120198 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

