Targeting the immunoproteasome in hypothalamic neurons as a novel therapeutic strategy for high-fat diet-induced obesity and metabolic dysregulation
- PMID: 39095788
- PMCID: PMC11297766
- DOI: 10.1186/s12974-024-03154-z
Targeting the immunoproteasome in hypothalamic neurons as a novel therapeutic strategy for high-fat diet-induced obesity and metabolic dysregulation
Abstract
Objective: Obesity represents a significant global health challenge characterized by chronic low-grade inflammation and metabolic dysregulation. The hypothalamus, a key regulator of energy homeostasis, is particularly susceptible to obesity's deleterious effects. This study investigated the role of the immunoproteasome, a specialized proteasomal complex implicated in inflammation and cellular homeostasis, during metabolic diseases.
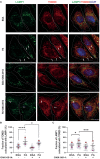
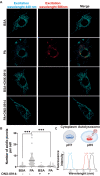
Methods: The levels of the immunoproteasome β5i subunit were analyzed by immunostaining, western blotting, and proteasome activity assay in mice fed with either a high-fat diet (HFD) or a regular diet (CHOW). We also characterized the impact of autophagy inhibition on the levels of the immunoproteasome β5i subunit and the activation of the AKT pathway. Finally, through confocal microscopy, we analyzed the contribution of β5i subunit inhibition on mitochondrial function by flow cytometry and mitophagy assay.
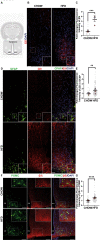
Results: Using an HFD-fed obese mouse model, we found increased immunoproteasome levels in hypothalamic POMC neurons. Furthermore, we observed that palmitic acid (PA), a major component of saturated fats found in HFD, increased the levels of the β5i subunit of the immunoproteasome in hypothalamic neuronal cells. Notably, the increase in immunoproteasome expression was associated with decreased autophagy, a critical cellular process in maintaining homeostasis and suppressing inflammation. Functionally, PA disrupted the insulin-glucose axis, leading to reduced AKT phosphorylation and increased intracellular glucose levels in response to insulin due to the upregulation of the immunoproteasome. Mechanistically, we identified that the protein PTEN, a key regulator of insulin signaling, was reduced in an immunoproteasome-dependent manner. To further investigate the potential therapeutic implications of these findings, we used ONX-0914, a specific immunoproteasome inhibitor. We demonstrated that this inhibitor prevents PA-induced insulin-glucose axis imbalance. Given the interplay between mitochondrial dysfunction and metabolic disturbances, we explored the impact of ONX-0914 on mitochondrial function. Notably, ONX-0914 preserved mitochondrial membrane potential and attenuated mitochondrial ROS production in the presence of PA. Moreover, we found that ONX-0914 reduced mitophagy in the presence of PA.
Conclusions: Our findings strongly support the pathogenic involvement of the immunoproteasome in hypothalamic neurons in the context of HFD-induced obesity and metabolic disturbances. Targeting the immunoproteasome highlights a promising therapeutic strategy to mitigate the detrimental effects of obesity on the insulin-glucose axis and cellular homeostasis. This study provides valuable insights into the mechanisms driving obesity-related metabolic diseases and offers potential avenues for developing novel therapeutic interventions.
Keywords: Autophagy; Hypothalamus; Immunoproteasome; Insulin-glucose axis; Metabolic disturbances; Mitochondrial function; Neurons; Obesity; Redox biology.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- von Frankenberg AD, Marina A, Song X, Callahan HS, Kratz M, Utzschneider KM. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur J Nutr. Feb. 2017;56(1):431–43. 10.1007/s00394-015-1108-6. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- FB210008/ANID/BASAL
- 21212138/ANID Doctoral Fellowship
- 21221618/ANID Doctoral Fellowship
- 1211261/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211261/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211261/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1221508/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1200965/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1230905/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211329/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1221508/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1240623/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211829/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211261/Fondo Nacional de Desarrollo Científico y Tecnológico
- ACT210039/ANILLO
- ACT172066/ANILLO
- ACT172066/ANILLO
- ACT172066/ANILLO
- ACT172066/ANILLO
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

