Toxoplasma infection induces an aged neutrophil population in the CNS that is associated with neuronal protection
- PMID: 39095837
- PMCID: PMC11297776
- DOI: 10.1186/s12974-024-03176-7
Toxoplasma infection induces an aged neutrophil population in the CNS that is associated with neuronal protection
Abstract
Background: Infection with the protozoan parasite Toxoplasma gondii leads to the formation of lifelong cysts in neurons that can have devastating consequences in the immunocompromised. In the immunocompetent individual, anti-parasitic effector mechanisms and a balanced immune response characterized by pro- and anti-inflammatory cytokine production establishes an asymptomatic infection that rarely leads to neurological symptoms. Several mechanisms are known to play a role in this successful immune response in the brain including T cell production of IFNγ and IL-10 and the involvement of CNS resident cells. This limitation of clinical neuropathology during chronic infection suggests a balance between immune response and neuroprotective mechanisms that collectively prevent clinical manifestations of disease. However, how these two vital mechanisms of protection interact during chronic Toxoplasma infection remains poorly understood.
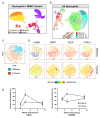
Main text: This study demonstrates a previously undescribed connection between innate neutrophils found chronically in the brain, termed "chronic brain neutrophils" (CBNeuts), and neuroprotective mechanisms during Toxoplasma infection. Lack of CBNeuts during chronic infection, accomplished via systemic neutrophil depletion, led to enhanced infection and deleterious effects on neuronal regeneration and repair mechanisms in the brain. Phenotypic and transcriptomic analysis of CBNeuts identified them as distinct from peripheral neutrophils and revealed two main subsets of CBNeuts that display heterogeneity towards both classical effector and neuroprotective functions in an age-dependent manner. Further phenotypic profiling defined expression of the neuroprotective molecules NRG-1 andErbB4 by these cells, and the importance of this signaling pathway during chronic infection was demonstrated via NRG-1 treatment studies.
Conclusions: In conclusion, this work identifies CBNeuts as a heterogenous population geared towards both classical immune responses and neuroprotection during chronic Toxoplasma infection and provides the foundation for future mechanistic studies of these cells.
Keywords: Toxoplasma gondii; Brain; Chronic infection; Immune response; Neuroprotection; Neutrophils.
© 2024. The Author(s).
Conflict of interest statement
BF holds patents related to the work being reported without direct corporate involvement at the time. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Montoya JG, Liesenfeld O, Toxoplasmosis. Lancet. 2004;363(9425):1965–76. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

