Injectable hydrogel encapsulating siMMP13 with anti-ROS and anti-apoptotic functions for osteoarthritis treatment
- PMID: 39095867
- PMCID: PMC11297633
- DOI: 10.1186/s12951-024-02740-w
Injectable hydrogel encapsulating siMMP13 with anti-ROS and anti-apoptotic functions for osteoarthritis treatment
Abstract
Background: Osteoarthritis (OA) is a degenerative joint disease characterized by the progressive degeneration of articular cartilage, leading to pain, stiffness, and loss of joint function. The pathogenesis of OA involves multiple factors, including increased intracellular reactive oxygen species (ROS), enhanced chondrocyte apoptosis, and disturbances in cartilage matrix metabolism. These processes contribute to the breakdown of the extracellular matrix (ECM) and the loss of cartilage integrity, ultimately resulting in joint damage and dysfunction. RNA interference (RNAi) therapy has emerged as a promising approach for the treatment of various diseases, including hATTR and acute hepatic porphyria. By harnessing the natural cellular machinery for gene silencing, RNAi allows for the specific inhibition of target genes involved in disease pathogenesis. In the context of OA, targeting key molecules such as matrix metalloproteinase-13 (MMP13), which plays a critical role in cartilage degradation, holds great therapeutic potential.
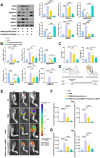
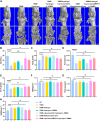
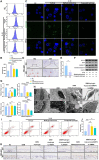
Results: In this study, we developed an innovative therapeutic approach for OA using a combination of liposome-encapsulated siMMP13 and NG-Monomethyl-L-arginine Acetate (L-NMMA) to form an injectable hydrogel. The hydrogel served as a delivery vehicle for the siMMP13, allowing for sustained release and targeted delivery to the affected joint. Experiments conducted on destabilization of the medial meniscus (DMM) model mice demonstrated the therapeutic efficacy of this composite hydrogel. Treatment with the hydrogel significantly inhibited the degradation of cartilage matrix, as evidenced by histological analysis showing preserved cartilage structure and reduced loss of proteoglycans. Moreover, the hydrogel effectively suppressed intracellular ROS accumulation in chondrocytes, indicating its anti-oxidative properties. Furthermore, it attenuated chondrocyte apoptosis, as demonstrated by decreased levels of apoptotic markers.
Conclusion: In summary, the injectable hydrogel containing siMMP13, endowed with anti-ROS and anti-apoptotic properties, may represent an effective therapeutic strategy for osteoarthritis in the future.
Keywords: Apoptosis; Cartilage; Chondrocyte; Hydrogel; Liposome; Osteoarthritis; RNAi; ROS; siMMP13.
© 2024. The Author(s).
Conflict of interest statement
The authors do not have competing interests to declare.
Figures





References
-
- Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–64. 10.1136/annrheumdis-2013-203355 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

