TIPE2 gene transfer ameliorates aging-associated osteoarthritis in a progeria mouse model by reducing inflammation and cellular senescence
- PMID: 39095992
- PMCID: PMC11403236
- DOI: 10.1016/j.ymthe.2024.07.027
TIPE2 gene transfer ameliorates aging-associated osteoarthritis in a progeria mouse model by reducing inflammation and cellular senescence
Abstract
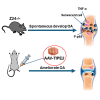
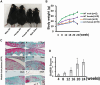
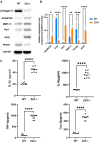
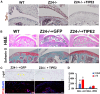
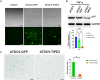
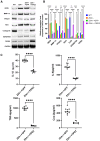
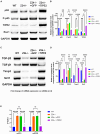
Osteoarthritis (OA) pain is often associated with the expression of tumor necrosis factor alpha (TNF-α), suggesting that TNF-α is one of the main contributing factors that cause inflammation, pain, and OA pathology. Thus, inhibition of TNF-α could potentially improve OA symptoms and slow disease progression. Anti-TNF-α treatments with antibodies, however, require multiple treatments and cannot entirely block TNF-α. TNF-α-induced protein 8-like 2 (TIPE2) was found to regulate the immune system's homeostasis and inflammation through different mechanisms from anti-TNF-α therapies. With a single treatment of adeno-associated virus (AAV)-TIPE2 gene delivery in the accelerated aging Zmpste24-/- (Z24-/-) mouse model, we found differences in Safranin O staining intensity within the articular cartilage (AC) region of the knee between TIPE2-treated mice and control mice. The glycosaminoglycan content (orange-red) was degraded in the Z24-/- cartilage while shown to be restored in the TIPE2-treated Z24-/- cartilage. We also observed that chondrocytes in Z24-/- mice exhibited a variety of senescent-associated phenotypes. Treatment with TIPE2 decreased TNF-α-positive cells, β-galactosidase (β-gal) activity, and p16 expression seen in Z24-/- mice. Our study demonstrated that AAV-TIPE2 gene delivery effectively blocked TNF-α-induced inflammation and senescence, resulting in the prevention or delay of knee OA in our accelerated aging Z24-/- mouse model.
Keywords: AAV; OA; TIPE2; adeno-associated virus; gene delivery; osteoarthritis; senescent cells; tumor necrosis factor α; tumor necrosis factor α-induced protein 8-like 2.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Li H., Xie S., Qi Y., Li H., Zhang R., Lian Y. TNF-alpha increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp. Ther. Med. 2018;16:4737–4744. doi: 10.3892/etm.2018.6770. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

