Solving the Etiology of Developmental and Epileptic Encephalopathy with Spike-Wave Activation in Sleep (D/EE-SWAS)
- PMID: 39096015
- PMCID: PMC11496008
- DOI: 10.1002/ana.27041
Solving the Etiology of Developmental and Epileptic Encephalopathy with Spike-Wave Activation in Sleep (D/EE-SWAS)
Abstract
Objective: To understand the etiological landscape and phenotypic differences between 2 developmental and epileptic encephalopathy (DEE) syndromes: DEE with spike-wave activation in sleep (DEE-SWAS) and epileptic encephalopathy with spike-wave activation in sleep (EE-SWAS).
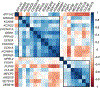
Methods: All patients fulfilled International League Against Epilepsy (ILAE) DEE-SWAS or EE-SWAS criteria with a Core cohort (n = 91) drawn from our Epilepsy Genetics research program, together with 10 etiologically solved patients referred by collaborators in the Expanded cohort (n = 101). Detailed phenotyping and analysis of molecular genetic results were performed. We compared the phenotypic features of individuals with DEE-SWAS and EE-SWAS. Brain-specific gene co-expression analysis was performed for D/EE-SWAS genes.
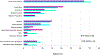
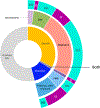
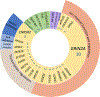
Results: We identified the etiology in 42/91 (46%) patients in our Core cohort, including 29/44 (66%) with DEE-SWAS and 13/47 (28%) with EE-SWAS. A genetic etiology was identified in 31/91 (34%). D/EE-SWAS genes were highly co-expressed in brain, highlighting the importance of channelopathies and transcriptional regulators. Structural etiologies were found in 12/91 (13%) individuals. We identified 10 novel D/EE-SWAS genes with a range of functions: ATP1A2, CACNA1A, FOXP1, GRIN1, KCNMA1, KCNQ3, PPFIA3, PUF60, SETD1B, and ZBTB18, and 2 novel copy number variants, 17p11.2 duplication and 5q22 deletion. Although developmental regression patterns were similar in both syndromes, DEE-SWAS was associated with a longer duration of epilepsy and poorer intellectual outcome than EE-SWAS.
Interpretation: DEE-SWAS and EE-SWAS have highly heterogeneous genetic and structural etiologies. Phenotypic analysis highlights valuable clinical differences between DEE-SWAS and EE-SWAS which inform clinical care and prognostic counseling. Our etiological findings pave the way for the development of precision therapies. ANN NEUROL 2024;96:932-943.
© 2024 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Potential conflicts of Interest
Nothing to report
Figures
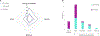
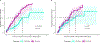

References
-
- Specchio N, Wirrell EC, Scheffer IE et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 63, 1398–442 (2022). - PubMed
-
- Caraballo R, Pavlidis E, Nikanorova M. et al. Encephalopathy with continuous spike-waves during slow-wave sleep: evolution and prognosis. Epileptic Disord. 21, 15–21 (2019). - PubMed
-
- Lesca G, Rudolf G, Bruneau N. et al. GRIN2A mutations in acquired epileptic aphasia and related childhood focal epilepsies and encephalopathies with speech and language dysfunction. Nat Genet. 45, 1061–6 (2013). - PubMed
-
- Lemke JR, Lal D, Reinthaler EM et al. Mutations in GRIN2A cause idiopathic focal epilepsy with rolandic spikes. Nat Genet. 45, 1067–72 (2013). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

