Neurofibrillary tangle-bearing neurons have reduced risk of cell death in mice with Alzheimer's pathology
- PMID: 39096489
- PMCID: PMC11441076
- DOI: 10.1016/j.celrep.2024.114574
Neurofibrillary tangle-bearing neurons have reduced risk of cell death in mice with Alzheimer's pathology
Abstract
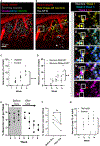
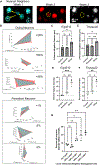
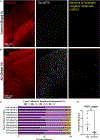
A prevailing hypothesis is that neurofibrillary tangles play a causal role in driving cognitive decline in Alzheimer's disease (AD) because tangles correlate anatomically with areas that undergo neuronal loss. We used two-photon longitudinal imaging to directly test this hypothesis and observed the fate of individual neurons in two mouse models. At any time point, neurons without tangles died at >3 times the rate as neurons with tangles. Additionally, prior to dying, they became >20% more distant from neighboring neurons across imaging sessions. Similar microstructural changes were evident in a population of non-tangle-bearing neurons in Alzheimer's donor tissues. Together, these data suggest that nonfibrillar tau puts neurons at high risk of death, and surprisingly, the presence of a tangle reduces this risk. Moreover, cortical microstructure changes appear to be a better predictor of imminent cell death than tangle status is and a promising tool for identifying dying neurons in Alzheimer's.
Keywords: Alzheimer’s disease; CP: Neuroscience; neurodegeneration; neurofibrillary tangles; tau; tauopathy; two-photon imaging.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests B.T.H. has a family member who works at Novartis and owns stock in Novartis; he serves on the SAB of Dewpoint and owns stock. He serves on a scientific advisory board or is a consultant for AbbVie, Avrobio, Axon, Biogen, BMS Cell Signaling, Genentech, Ionis, Novartis, Seer, Takeda, the United States Department of Justice, and Vigil, Voyager. His laboratory is supported by sponsored research agreements with AbbVie and F Prime and research grants from the Cure Alzheimer’s Fund, Tau Consortium, and the JPB Foundation. R.E.B. works on the AbbVie-Hyman Collaboration.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

