Co-observation of germline pathogenic variants in breast cancer predisposition genes: Results from analysis of the BRIDGES sequencing dataset
- PMID: 39096911
- PMCID: PMC11393698
- DOI: 10.1016/j.ajhg.2024.07.004
Co-observation of germline pathogenic variants in breast cancer predisposition genes: Results from analysis of the BRIDGES sequencing dataset
Abstract
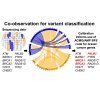
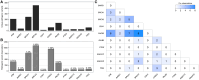
Co-observation of a gene variant with a pathogenic variant in another gene that explains the disease presentation has been designated as evidence against pathogenicity for commonly used variant classification guidelines. Multiple variant curation expert panels have specified, from consensus opinion, that this evidence type is not applicable for the classification of breast cancer predisposition gene variants. Statistical analysis of sequence data for 55,815 individuals diagnosed with breast cancer from the BRIDGES sequencing project was undertaken to formally assess the utility of co-observation data for germline variant classification. Our analysis included expected loss-of-function variants in 11 breast cancer predisposition genes and pathogenic missense variants in BRCA1, BRCA2, and TP53. We assessed whether co-observation of pathogenic variants in two different genes occurred more or less often than expected under the assumption of independence. Co-observation of pathogenic variants in each of BRCA1, BRCA2, and PALB2 with the remaining genes was less frequent than expected. This evidence for depletion remained after adjustment for age at diagnosis, study design (familial versus population-based), and country. Co-observation of a variant of uncertain significance in BRCA1, BRCA2, or PALB2 with a pathogenic variant in another breast cancer gene equated to supporting evidence against pathogenicity following criterion strength assignment based on the likelihood ratio and showed utility in reclassification of missense BRCA1 and BRCA2 variants identified in BRIDGES. Our approach has applicability for assessing the value of co-observation as a predictor of variant pathogenicity in other clinical contexts, including for gene-specific guidelines developed by ClinGen Variant Curation Expert Panels.
Keywords: ACMG/AMP; BP5; breast cancer; co-observation; genetics; sequencing data; variant classification.
Copyright © 2024 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.A.F. conducts research funded by Amgen, Novartis, and Pfizer. He received Honoraria from Roche, Novartis, and Pfizer. A.R.M. received funds from AstraZeneca for contribution to sponsored quality assessments and variant interpretation of VUSs in BRCA1 and BRCA2. The funds were paid to the institution.
Figures
References
-
- Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. - PMC - PubMed
-
- Luo X., Maciaszek J.L., Thompson B.A., Leong H.S., Dixon K., Sousa S., Anderson M., Roberts M.E., Lee K., Spurdle A.B., et al. Optimising clinical care through CDH1-specific germline variant curation: improvement of clinical assertions and updated curation guidelines. J. Med. Genet. 2022;60:568–575. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

