Prevalence of anti-HLA antibodies in COVID-19 convalescent plasma donors: an Indian experience
- PMID: 39097433
- PMCID: PMC11451383
- DOI: 10.1016/j.htct.2024.03.003
Prevalence of anti-HLA antibodies in COVID-19 convalescent plasma donors: an Indian experience
Abstract
Background: COVID-19 convalescent plasma is one of the experimental therapies used widely in moderately sick COVID-19 patients. However, there are a few risks involved in plasma transfusion; notably, transfusion-related acute lung injury (TRALI) caused by antibodies against human leukocyte antigens (HLA). This study was designed to assess the prevalence of anti-HLA antibodies in convalescent plasma donors using the single antigen bead method.
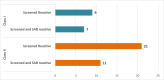
Study design and methods: This was a hospital-based observational study of consecutive plasma donors. A total of 252 samples were screened for anti-HLA Class I and Class II antibodies using the microbead assay with the identification of anti-HLA Ab in positive samples being performed using a single antigen bead assay. Luminex-based normalized background cutoff ratios of 10.8 for Class I and 6.9 for Class II and mean fluorescence intensity cutoffs of 2500 for Class I and 1500 for Class II were used for screening and the single bead assay, respectively.
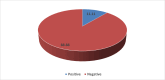
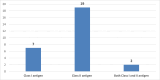
Results: Of 252 screened samples, 28 (11.1 %) were positive for Class I, Class II or both Class I and Class II anti-HLA antibodies in donors with no history of a previous immunizing event. Moreover, 20/252 (7.9%) donors without any history of prior immunization had specific anti-HLA antibodies of Class I or Class II or both by the single bead assay.
Conclusions: The high prevalence of anti-HLA antibodies in our cohort of donors raises an urgent and immediate need for anti-HLA antibody screening in all convalescent plasma donors for safe therapy of COVID-19 patients.
Keywords: Anti HLA antibody; CCP; TRALI.
Copyright © 2024. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors confirm that there are no conflicts of interest.
Figures
References
-
- Semple J.W., Rebetz J., Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133(17):1840–1853. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

