Translational T-box riboswitches bind tRNA by modulating conformational flexibility
- PMID: 39097611
- PMCID: PMC11297988
- DOI: 10.1038/s41467-024-50885-x
Translational T-box riboswitches bind tRNA by modulating conformational flexibility
Abstract
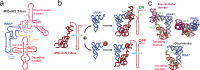
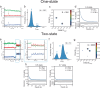
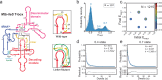
T-box riboswitches are noncoding RNA elements involved in genetic regulation of most Gram-positive bacteria. They regulate amino acid metabolism by assessing the aminoacylation status of tRNA, subsequently affecting the transcription or translation of downstream amino acid metabolism-related genes. Here we present single-molecule FRET studies of the Mycobacterium tuberculosis IleS T-box riboswitch, a paradigmatic translational T-box. Results support a two-step binding model, where the tRNA anticodon is recognized first, followed by interactions with the NCCA sequence. Furthermore, after anticodon recognition, tRNA can transiently dock into the discriminator domain even in the absence of the tRNA NCCA-discriminator interactions. Establishment of the NCCA-discriminator interactions significantly stabilizes the fully bound state. Collectively, the data suggest high conformational flexibility in translational T-box riboswitches; and supports a conformational selection model for NCCA recognition. These findings provide a kinetic framework to understand how specific RNA elements underpin the binding affinity and specificity required for gene regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

