Effect of pneumococcal conjugate vaccine six years post-introduction on pneumococcal carriage in Ulaanbaatar, Mongolia
- PMID: 39097620
- PMCID: PMC11297977
- DOI: 10.1038/s41467-024-50944-3
Effect of pneumococcal conjugate vaccine six years post-introduction on pneumococcal carriage in Ulaanbaatar, Mongolia
Abstract
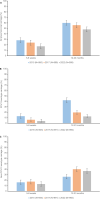
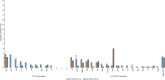
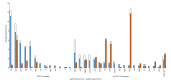
Limited data from Asia are available on long-term effects of pneumococcal conjugate vaccine introduction on pneumococcal carriage. Here we assess the impact of 13-valent pneumococcal conjugate vaccine (PCV13) introduction on nasopharyngeal pneumococcal carriage prevalence, density and antimicrobial resistance. Cross-sectional carriage surveys were conducted pre-PCV13 (2015) and post-PCV13 introduction (2017 and 2022). Pneumococci were detected and quantified by real-time PCR from nasopharyngeal swabs. DNA microarray was used for molecular serotyping and to infer genetic lineage (Global Pneumococcal Sequence Cluster). The study included 1461 infants (5-8 weeks old) and 1489 toddlers (12-23 months old) enrolled from family health clinics. We show a reduction in PCV13 serotype carriage (with non-PCV13 serotype replacement) and a reduction in the proportion of samples containing resistance genes in toddlers six years post-PCV13 introduction. We observed an increase in pneumococcal nasopharyngeal density. Serotype 15 A, the most prevalent non-vaccine-serotype in 2022, was comprised predominantly of GPSC904;9. Reductions in PCV13 serotype carriage will likely result in pneumococcal disease reduction. It is important for ongoing surveillance to monitor serotype changes to potentially inform new vaccine development.
© 2024. The Author(s).
Conflict of interest statement
C.V.M., M.U., C.D.N., P.B., B.S., D.L., C.S., T.M., and E.K.M. are investigators on a Pfizer collaborative research project, exploring the impact of PCV13 introduction on adult pneumonia in Mongolia, outside this work. E.M.D. is currently employed by Pfizer. C.S., E.K.M., and C.D.N. are investigators on a Merck Investigator Studies Program grant funded by MSD outside this work. C.V.M. has participated in expert forums for Pfizer and MSD. C.S. has participated in forums and seminars for Pfizer and MSD. J.H. is co-founder and shareholder of BUGS Bioscience Ltd., a not-for-profit spin-out company of St George’s, University of London. The remaining authors declare no competing interest.
Figures
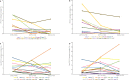

References
-
- International Vaccine Access Center (IVAC), Johns Hopkins Bloomberg School of Public Health. VIEW-hub 2023. Available from: www.view-hub.org. (Accessed 03 April 2023).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

