Compositional profiling of the rhizosphere microbiome of Canada thistle reveals consistent patterns across the United States northern Great Plains
- PMID: 39097653
- PMCID: PMC11298000
- DOI: 10.1038/s41598-024-69082-3
Compositional profiling of the rhizosphere microbiome of Canada thistle reveals consistent patterns across the United States northern Great Plains
Abstract
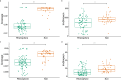
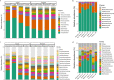
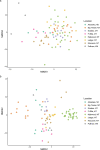
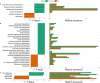
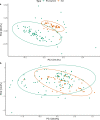
Canada thistle is a pervasive perennial weed, causing challenges to agricultural and natural ecosystems globally. Although research has focused on the phenology, genetics, and control of Canada thistle, little is known about the rhizosphere microbiome and the role plant-microbe interactions play in invasion success. This study investigated the rhizosphere microbiome of Canada thistle across diverse climates, soils, and crops in the U.S. northern Great Plains. Soil and rhizosphere samples were collected and bacterial 16S and fungal ITS2 sequencing were performed to characterize the core microbiome and identify potential factors contributing to invasion success. Amplicon sequencing revealed a stable core microbiome that was detected in the Canada thistle rhizosphere across all locations. The core microbiome was dominated by the bacterial phyla Actinobacteriota and Proteobacteria and fungal phyla Ascomycota and Basidiomycota. Differential abundance analysis showed rhizosphere fungal communities were enriched in pathogen-containing genera with a 1.7-fold greater abundance of Fusaria and a 2.6-fold greater abundance of Gibberella compared to bulk soil. Predictive functional profiling showed rhizosphere communities were enriched (p < 0.05, FDR corrected) in plant pathogen fungal guilds which represented 19% of the fungal community. The rhizosphere microbiome was similar in composition across environments, highlighting the stable association between Canada thistle and specific microbial taxa. This study characterized the core microbiome of Canada thistle, and the findings highlight plant-microbe interactions shaping invasive behavior. These findings are important for understanding the ecological impacts of plant invasion and soil-microbe ecological processes.
Keywords: Canada thistle; Invasive species; Rhizosphere microbiome; Weeds.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cripps, M. G. et al. Classical biological control of Cirsium arvense: Lessons from the past. Biol. Control57, 165–174. 10.1016/j.biocontrol.2011.03.011 (2011).10.1016/j.biocontrol.2011.03.011 - DOI
-
- Hodgins, K. A., Guggisberg, A., Nurkowski, K. & Rieseberg, L. H. Genetically based trait differentiation but lack of trade-offs between stress tolerance and performance in introduced Canada thistle. Plant Commun.1, 100116. 10.1016/j.xplc.2020.100116 (2020). 10.1016/j.xplc.2020.100116 - DOI - PMC - PubMed
-
- Katovich, E., Becker, R., Chandler, M. & Marek-Spartz, M. Biological control of Canada thistle (Cirsium arvense) revisited: Host range of Hadroplontus litura on Cirsium species native to the Upper Midwest, USA. Biocontrol Sci. Technol.32, 1050–1064. 10.1080/09583157.2022.2085245 (2022).10.1080/09583157.2022.2085245 - DOI
-
- Hodgson, J. M. The response of Canada thistle ecotypes to 2,4-D, amitrole, and intensive cultivation. Weed Sci.18, 253–255. 10.1017/S0043174500079686 (1970).10.1017/S0043174500079686 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

