Immunogenicity and biodistribution of lipid nanoparticle formulated self-amplifying mRNA vaccines against H5 avian influenza
- PMID: 39097672
- PMCID: PMC11298010
- DOI: 10.1038/s41541-024-00932-x
Immunogenicity and biodistribution of lipid nanoparticle formulated self-amplifying mRNA vaccines against H5 avian influenza
Abstract
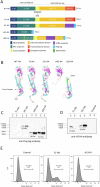
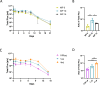
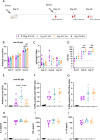
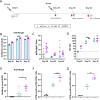
This study reports on the immunogenicity and biodistribution of H5 hemagglutinin (HA)-based self-amplifying (sa) mRNA vaccines in mice. Four sa-mRNA vaccines encoding either a secreted full-length HA, a secreted HA head domain, a secreted HA stalk domain, or a full-length membrane-anchored HA were investigated. All vaccines elicited an adaptive immune response. However, the full-length HA sa-RNA vaccines demonstrated superior performance compared to head and stalk domain vaccines. The antibody titers positively correlated with the vaccine dose. Cellular immune responses and antigen-specific IgA antibodies in the lungs were also observed. The comparison of the sa-mRNA vaccines encoding the secreted and membrane-anchored full-length HA revealed that anchoring of the HA to the membrane significantly enhanced the antibody and cellular responses. In addition to the injection site, the intramuscularly injected sa-mRNA-LNPs were also detected in the draining lymph nodes, spleen, and to a lesser extent, in the lung, kidney, liver, and heart.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Chen, H. & Bu, Z. Development and application of avian influenza vaccines in China. Curr. Top. Microbiol. Immunol.333, 153–162 (2009). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

