Utilizing deep learning model for assessing melanocytic density in resection margins of lentigo maligna
- PMID: 39097745
- PMCID: PMC11297622
- DOI: 10.1186/s13000-024-01532-y
Utilizing deep learning model for assessing melanocytic density in resection margins of lentigo maligna
Erratum in
-
Correction: Utilizing deep learning model for assessing melanocytic density in resection margins of lentigo maligna.Diagn Pathol. 2024 Sep 5;19(1):119. doi: 10.1186/s13000-024-01545-7. Diagn Pathol. 2024. PMID: 39238025 Free PMC article. No abstract available.
Abstract
Background: Surgical excision with clear histopathological margins is the preferred treatment to prevent progression of lentigo maligna (LM) to invasive melanoma. However, the assessment of resection margins on sun-damaged skin is challenging. We developed a deep learning model for detection of melanocytes in resection margins of LM.
Methods: In total, 353 whole slide images (WSIs) were included. 295 WSIs were used for training and 58 for validation and testing. The algorithm was trained with 3,973 manual pixel-wise annotations. The AI analyses were compared to those of three blinded dermatopathologists and two pathology residents, who performed their evaluations without AI and AI-assisted. Immunohistochemistry (SOX10) served as the reference standard. We used a dichotomized cutoff for low and high risk of recurrence (≤ 25 melanocytes in an area of 0.5 mm for low risk and > 25 for high risk).
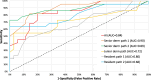
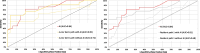
Results: The AI model achieved an area under the receiver operating characteristic curve (AUC) of 0.84 in discriminating margins with low and high recurrence risk. In comparison, the AUC for dermatopathologists ranged from 0.72 to 0.90 and for the residents in pathology, 0.68 to 0.80. Additionally, with aid of the AI model the performance of two pathologists significantly improved.
Conclusions: The deep learning showed notable accuracy in detecting resection margins of LM with a high versus low risk of recurrence. Furthermore, the use of AI improved the performance of 2/5 pathologists. This automated tool could aid pathologists in the assessment or pre-screening of LM margins.
Keywords: Computational pathology; Deep learning; Lentigo maligna; Margin assessment; Melanocytic count.
© 2024. The Author(s).
Conflict of interest statement
Darshan Kumar is an employee of Aiforia Technolocies Plc. Noora Neittaanmäki has received consultation fees from Aiforia Technologies Plc.
Figures

References
-
- Barlow JO, Maize J Sr, Lang PG. The density and distribution of melanocytes adjacent to melanoma and nonmelanoma skin cancers. Dermatol Surg. 2007;33:199–207. - PubMed
-
- Weyers W, Bonczkowitz M, Weyers I, Bittinger A, Schill WB. Melanoma in situ versus melanocytic hyperplasia in sun-damaged skin. Assessment of the significance of histopathologic criteria for differential diagnosis. Am J Dermatopathol. 1996;18(6):560–6. 10.1097/00000372-199612000-00002 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

